Figure 5. Effect of fish oil on AP-2, Sp1 and NF-κB binding activities.
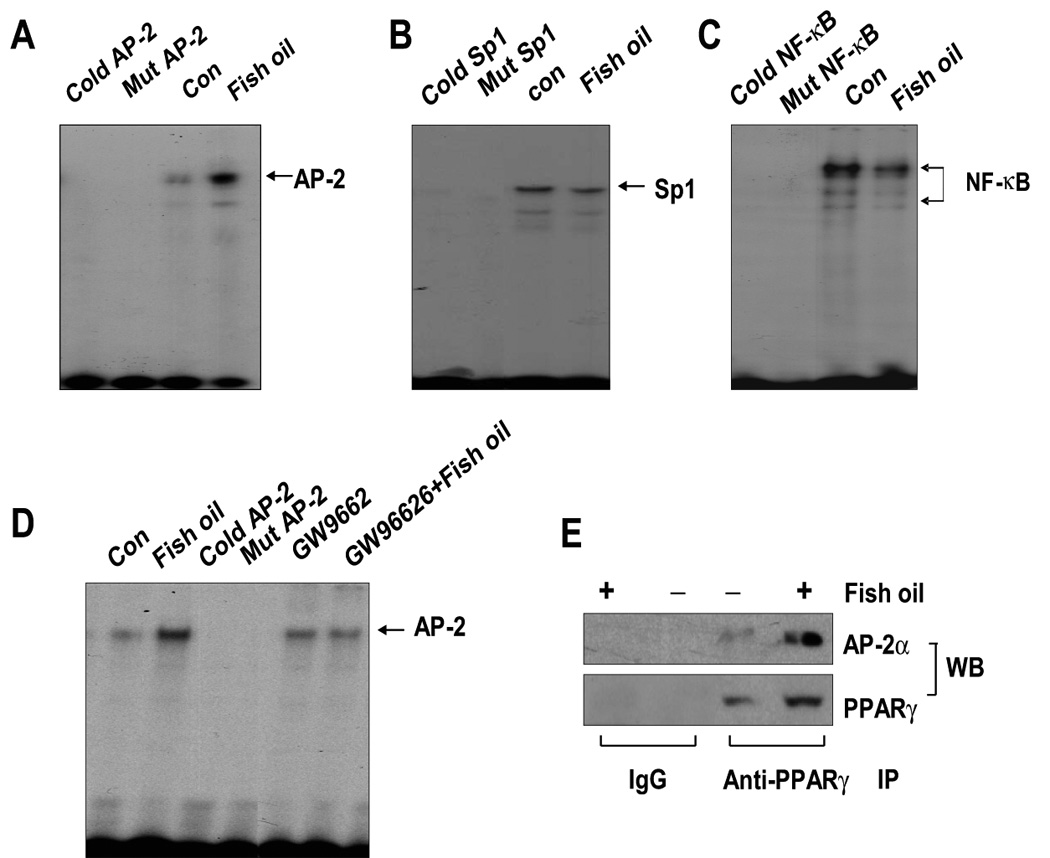
A, Oligonucleotides containing the AP-2 (A), Sp1 (B) and NF-κB (C) sites were end-labeled with [γ-32P]-ATP and incubated with nuclear extracts (5 µg) from H1838 cells treated with fish oil (10 µg/ml) for an additional 24 h. For competition assays, a molar excess (×100) of consensus Sp1 (Cold Sp1) or NF-κB (Cold NF-κB) or AP-2 (Cold AP-2) oligonucleotide were added to the binding reaction. Oligonucleotides containing a mutated Sp1 (Mut Sp1) or NF-κB (Mut NF-κB) or AP-2 (Mut AP-2) site that were end-labeled with γ32P-ATP were used to confirm the binding specificity. Con, indicates untreated control cells. D, Oligonucleotides containing the AP-2 site was end-labeled with [γ-32P]-ATP and incubated with nuclear extracts (5 µg) from H1838 cells treated with GW9662 (20 µM) for 2 h before exposure of the cells to fish oil (10 µg/ml) for an additional 24 h. For competition assays, a molar excess (×100) of consensus AP-2 (Cold AP-2) oligonucleotide was added to the binding reaction. Oligonucleotides containing a mutated AP-2 (Mut AP-2) site that was end labeled with γ32P-ATP were used to confirm the binding specificity. Con, indicates untreated control cells. E, H1838 cells were treated with fish oil (10 µg/ml) for 24 h and cell lysates were immunoprecipitated with either IgG, or anti-PPARγ. Immunoprecipitates were separated by SDS-PAGE in 10% Tris-glycine gels, transferred onto nitrocellulose and Western blotting (WB) was carried out with either anti-PPARγ or anti-AP-2α antibody.
