Abstract
Genetic determinants of the invasive phenotype of Shigella spp. and enteroinvasive Escherichia coli (EIEC), two common agents of bacillary dysentery, are encoded on large (180- to 210 kilobase), nonconjugative plasmids. Several plasmid-encoded antigens have been implicated as important bacterial ligands that mediate the attachment and invasion of colonic epithelial cells by the bacteria. Selected invasion plasmid antigen (ipa) genes have recently been cloned from Shigella flexneri serotype 5 into the lambda gt11 expression vector. Portions of three ipa genes (ipaB, ipaC, and ipaD) were tested as DNA probes for diagnostic detection of bacillary dysentery. Under stringent DNA hybridization conditions, all three DNA sequences hybridized to a single 4.6-kilobase HindIII fragment of the invasion plasmids of representative virulent Shigella spp. and EIEC strains. No hybridization was detected in isogenic, noninvasive Shigella mutants which had lost the invasion plasmid or had deleted the ipa gene region. Furthermore, these probes did not react with over 300 other enteric and nonenteric gram-negative bacteria tested, including Salmonella, Yersinia, Edwardsiella, Campylobacter, Vibrio, Klebsiella, Aeromonas, Enterobacter, Rickettsia, and Citrobacter spp. and various pathogenic E. coli strains. The use of unique invasion-essential gene segments as probes for the specific detection of invasive dysentery organisms should benefit both epidemiologic and diagnostic analyses of Shigella spp. and EIEC.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boileau C. R., d'Hauteville H. M., Sansonetti P. J. DNA hybridization technique to detect Shigella species and enteroinvasive escherichia coli. J Clin Microbiol. 1984 Nov;20(5):959–961. doi: 10.1128/jcm.20.5.959-961.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
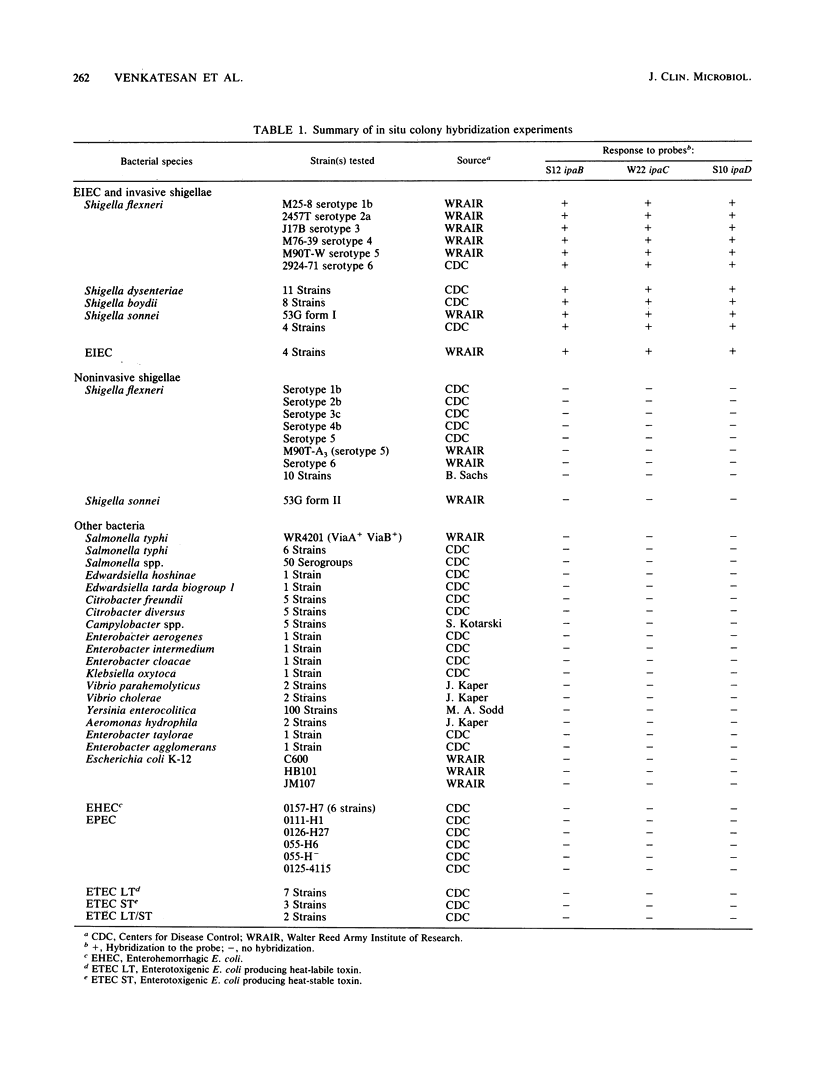
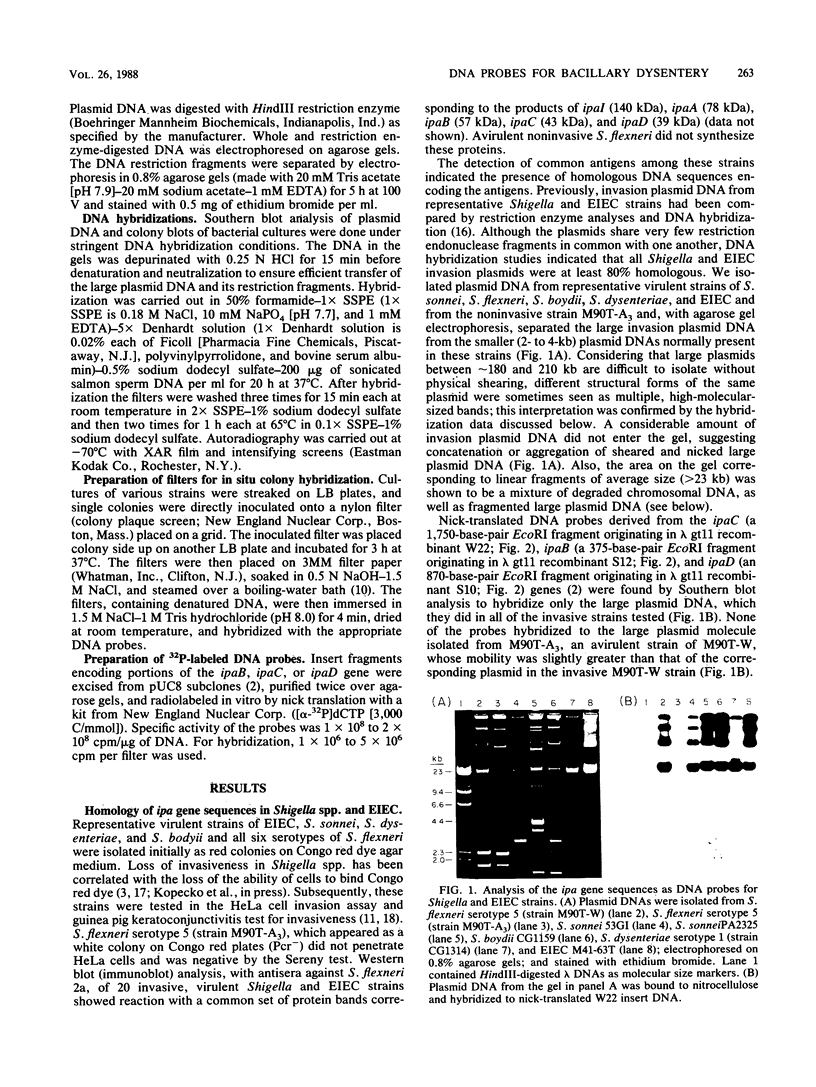
- Buysse J. M., Stover C. K., Oaks E. V., Venkatesan M., Kopecko D. J. Molecular cloning of invasion plasmid antigen (ipa) genes from Shigella flexneri: analysis of ipa gene products and genetic mapping. J Bacteriol. 1987 Jun;169(6):2561–2569. doi: 10.1128/jb.169.6.2561-2569.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
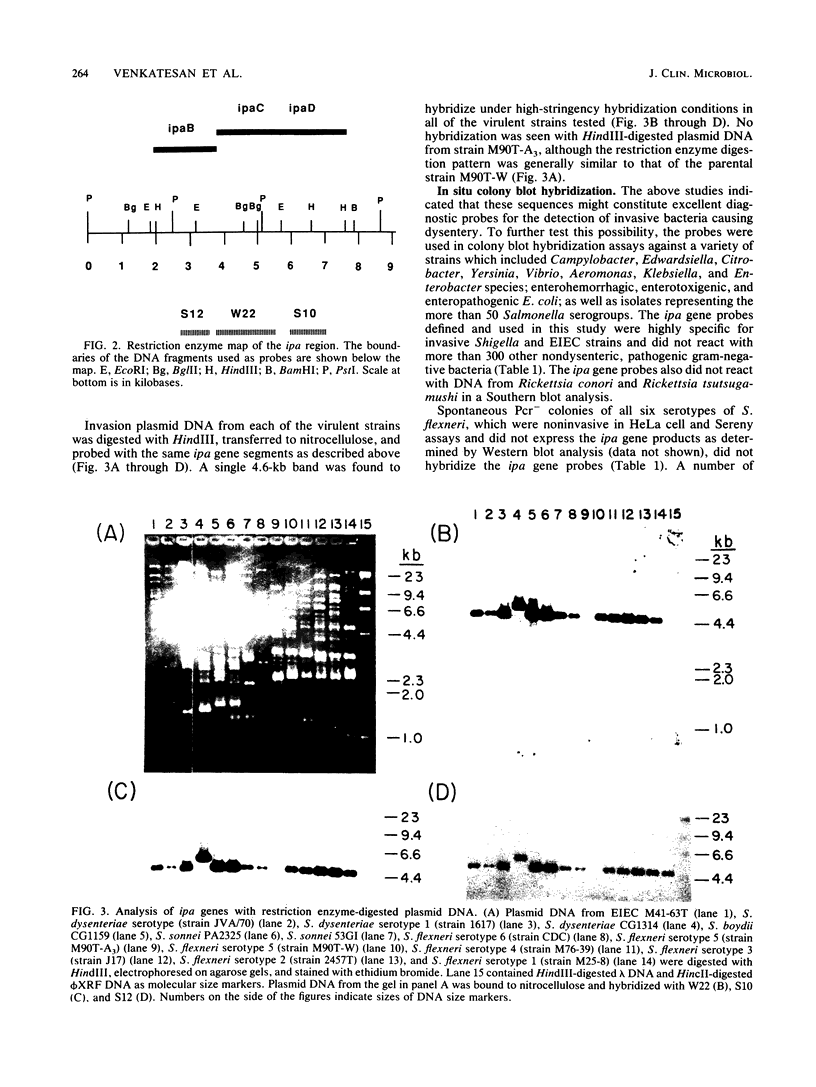
- Daskaleros P. A., Payne S. M. Characterization of Shigella flexneri sequences encoding congo red binding (crb): conservation of multiple crb sequences and role of IS1 in loss of the Crb+ phenotype. Infect Immun. 1986 Nov;54(2):435–443. doi: 10.1128/iai.54.2.435-443.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinari G., Hale T. L., Austin S. W., Formal S. B. Local and systemic antibody responses to Shigella infection in rhesus monkeys. J Infect Dis. 1987 May;155(5):1065–1069. doi: 10.1093/infdis/155.5.1065. [DOI] [PubMed] [Google Scholar]
- Hale T. L., Formal S. B. Genetics of virulence in Shigella. Microb Pathog. 1986 Dec;1(6):511–518. doi: 10.1016/0882-4010(86)90037-9. [DOI] [PubMed] [Google Scholar]
- Hale T. L., Oaks E. V., Formal S. B. Identification and antigenic characterization of virulence-associated, plasmid-coded proteins of Shigella spp. and enteroinvasive Escherichia coli. Infect Immun. 1985 Dec;50(3):620–629. doi: 10.1128/iai.50.3.620-629.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale T. L., Sansonetti P. J., Schad P. A., Austin S., Formal S. B. Characterization of virulence plasmids and plasmid-associated outer membrane proteins in Shigella flexneri, Shigella sonnei, and Escherichia coli. Infect Immun. 1983 Apr;40(1):340–350. doi: 10.1128/iai.40.1.340-350.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopecko D. J., Baron L. S., Buysse J. Genetic determinants of virulence in Shigella and dysenteric strains of Escherichia coli: their involvement in the pathogenesis of dysentery. Curr Top Microbiol Immunol. 1985;118:71–95. doi: 10.1007/978-3-642-70586-1_5. [DOI] [PubMed] [Google Scholar]
- Labrec E. H., Schneider H., Magnani T. J., Formal S. B. EPITHELIAL CELL PENETRATION AS AN ESSENTIAL STEP IN THE PATHOGENESIS OF BACILLARY DYSENTERY. J Bacteriol. 1964 Nov;88(5):1503–1518. doi: 10.1128/jb.88.5.1503-1518.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maas R. An improved colony hybridization method with significantly increased sensitivity for detection of single genes. Plasmid. 1983 Nov;10(3):296–298. doi: 10.1016/0147-619x(83)90045-8. [DOI] [PubMed] [Google Scholar]
- Maurelli A. T., Baudry B., d'Hauteville H., Hale T. L., Sansonetti P. J. Cloning of plasmid DNA sequences involved in invasion of HeLa cells by Shigella flexneri. Infect Immun. 1985 Jul;49(1):164–171. doi: 10.1128/iai.49.1.164-171.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyman K., Nakamura K., Ohtsubo H., Ohtsubo E. Distribution of the insertion sequence IS1 in gram-negative bacteria. Nature. 1981 Feb 12;289(5798):609–612. doi: 10.1038/289609a0. [DOI] [PubMed] [Google Scholar]
- Oaks E. V., Hale T. L., Formal S. B. Serum immune response to Shigella protein antigens in rhesus monkeys and humans infected with Shigella spp. Infect Immun. 1986 Jul;53(1):57–63. doi: 10.1128/iai.53.1.57-63.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal T., Echeverria P., Taylor D. N., Sethabutr O., Hanchalay S. Identification of enteroinvasive Escherichia coli by indirect ELISA and DNA hybridisation. Lancet. 1985 Oct 5;2(8458):785–785. doi: 10.1016/s0140-6736(85)90669-5. [DOI] [PubMed] [Google Scholar]
- SERENY B. Experimental shigella keratoconjunctivitis; a preliminary report. Acta Microbiol Acad Sci Hung. 1955;2(3):293–296. [PubMed] [Google Scholar]
- Sakai T., Sasakawa C., Makino S., Yoshikawa M. DNA sequence and product analysis of the virF locus responsible for congo red binding and cell invasion in Shigella flexneri 2a. Infect Immun. 1986 Nov;54(2):395–402. doi: 10.1128/iai.54.2.395-402.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasakawa C., Kamata K., Sakai T., Murayama S. Y., Makino S., Yoshikawa M. Molecular alteration of the 140-megadalton plasmid associated with loss of virulence and Congo red binding activity in Shigella flexneri. Infect Immun. 1986 Feb;51(2):470–475. doi: 10.1128/iai.51.2.470-475.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sethabutr O., Hanchalay S., Echeverria P., Taylor D. N., Leksomboon U. A non-radioactive DNA probe to identify Shigella and enteroinvasive Escherichia coli in stools of children with diarrhoea. Lancet. 1985 Nov 16;2(8464):1095–1097. doi: 10.1016/s0140-6736(85)90687-7. [DOI] [PubMed] [Google Scholar]
- Watanabe H., Nakamura A. Identification of Shigella sonnei form I plasmid genes necessary for cell invasion and their conservation among Shigella species and enteroinvasive Escherichia coli. Infect Immun. 1986 Aug;53(2):352–358. doi: 10.1128/iai.53.2.352-358.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood P. K., Morris J. G., Jr, Small P. L., Sethabutr O., Toledo M. R., Trabulsi L., Kaper J. B. Comparison of DNA probes and the Sereny test for identification of invasive Shigella and Escherichia coli strains. J Clin Microbiol. 1986 Sep;24(3):498–500. doi: 10.1128/jcm.24.3.498-500.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]