Abstract
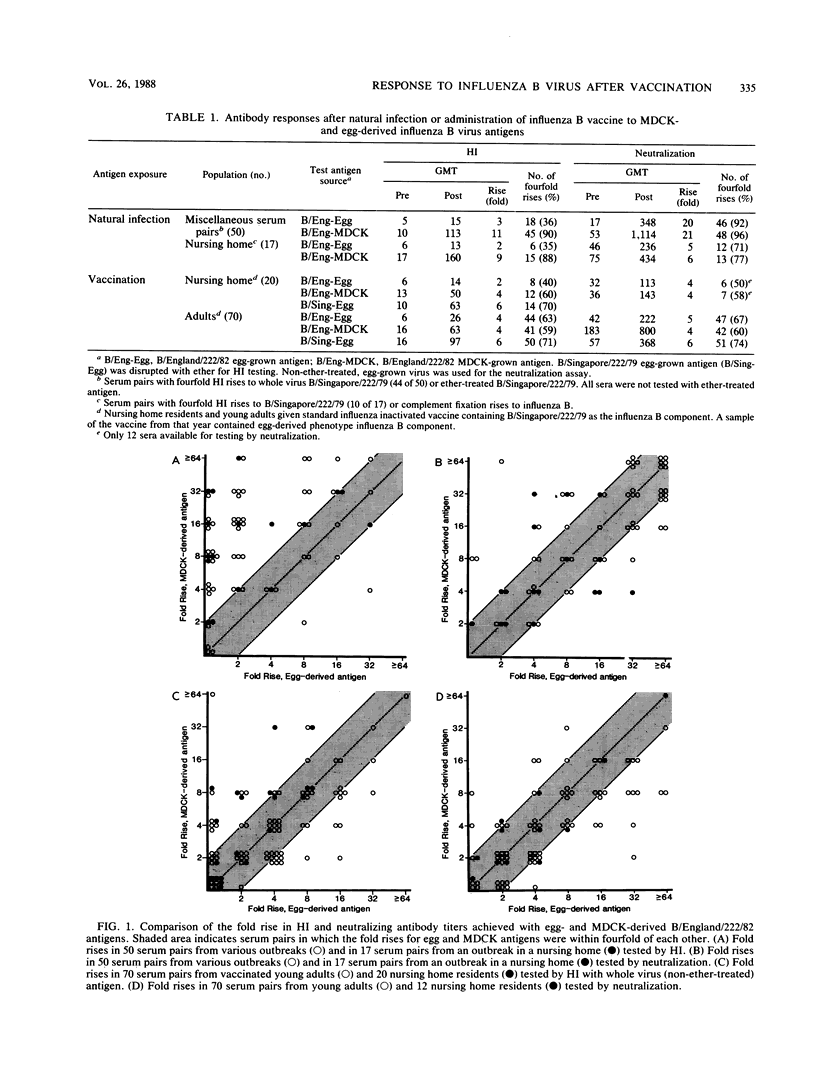
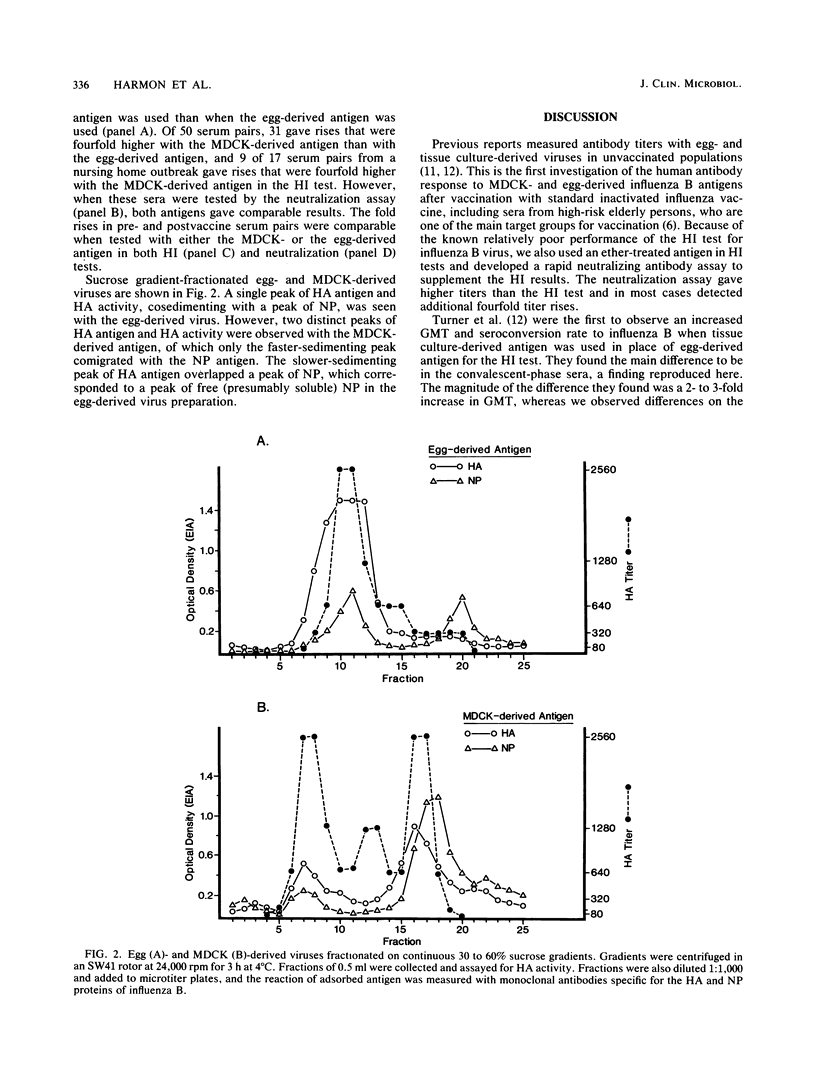
Hemagglutination inhibition (HI) and neutralization tests were used to determine antibody responses to egg-derived and Madin-Darby canine kidney (MDCK)-derived influenza B virus (B/England/222/82) in paired sera from persons naturally infected with influenza B and in persons vaccinated with standard egg-derived inactivated influenza vaccine. When tested by HI, the MDCK-derived antigen gave significantly higher (8- to 12-fold) geometric mean titers (GMT) in convalescent-phase sera from persons naturally infected during community outbreaks, as well as more 4-fold titer rises, than did tests with egg-derived antigen. When tested by neutralization, however, the convalescent-phase sera GMTs were only threefold higher with the MDCK-derived antigen and an equivalent number of fourfold titer rises were detected with both antigens. With postvaccine sera, the MDCK-derived antigen gave GMTs that were threefold higher than those obtained with egg-derived antigen in both the HI and neutralization tests and both antigens detected an equivalent number of fourfold titer rises in HI and neutralization tests. Sucrose gradient-fractionated egg-derived antigen showed a single peak of hemagglutinin activity corresponding to whole virions, whereas MDCK-derived antigen contained two distinct peaks of hemagglutinin activity, one of which had a lower sedimentation rate. The overall findings indicate that the egg-derived antigen in the vaccine induced HI and neutralizing antibody to both egg- and MDCK-derived variants and suggest that titers of antibody to MDCK-derived virus may be affected by the physical form of the hemagglutinin antigen.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson L. J., Hierholzer J. C., Bingham P. G., Stone Y. O. Microneutralization test for respiratory syncytial virus based on an enzyme immunoassay. J Clin Microbiol. 1985 Dec;22(6):1050–1052. doi: 10.1128/jcm.22.6.1050-1052.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank A. L., Puck J., Hughes B. J., Cate T. R. Microneutralization test for influenza A and B and parainfluenza 1 and 2 viruses that uses continuous cell lines and fresh serum enhancement. J Clin Microbiol. 1980 Sep;12(3):426–432. doi: 10.1128/jcm.12.3.426-432.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendal A. P., Noble G. R., Dowdle W. R. Swine influenza viruses isolated in 1976 from man and pig contain two coexisting subpopulations with antigenically distinguishable hemagglutinins. Virology. 1977 Oct 1;82(1):111–121. doi: 10.1016/0042-6822(77)90037-x. [DOI] [PubMed] [Google Scholar]
- Kilbourne E. D. Genetic dimorphism in influenza viruses: characterization of stably associated hemagglutinin mutants differing in antigenicity and biological properties. Proc Natl Acad Sci U S A. 1978 Dec;75(12):6258–6262. doi: 10.1073/pnas.75.12.6258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monto A. S., Maassab H. F. Ether treatment of type B influenza virus antigen for the hemagglutination inhibition test. J Clin Microbiol. 1981 Jan;13(1):54–57. doi: 10.1128/jcm.13.1.54-57.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson J. S., Naeve C. W., Webster R. G., Bootman J. S., Newman R., Schild G. C. Alterations in the hemagglutinin associated with adaptation of influenza B virus to growth in eggs. Virology. 1985 May;143(1):166–174. doi: 10.1016/0042-6822(85)90105-9. [DOI] [PubMed] [Google Scholar]
- Schild G. C., Oxford J. S., de Jong J. C., Webster R. G. Evidence for host-cell selection of influenza virus antigenic variants. Nature. 1983 Jun 23;303(5919):706–709. doi: 10.1038/303706a0. [DOI] [PubMed] [Google Scholar]
- Turner R., Lathey J. L., Van Voris L. P., Belshe R. B. Serological diagnosis of influenza B virus infection: comparison of an enzyme-linked immunosorbent assay and the hemagglutination inhibition test. J Clin Microbiol. 1982 May;15(5):824–829. doi: 10.1128/jcm.15.5.824-829.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walls H. H., Harmon M. W., Slagle J. J., Stocksdale C., Kendal A. P. Characterization and evaluation of monoclonal antibodies developed for typing influenza A and influenza B viruses. J Clin Microbiol. 1986 Feb;23(2):240–245. doi: 10.1128/jcm.23.2.240-245.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]


