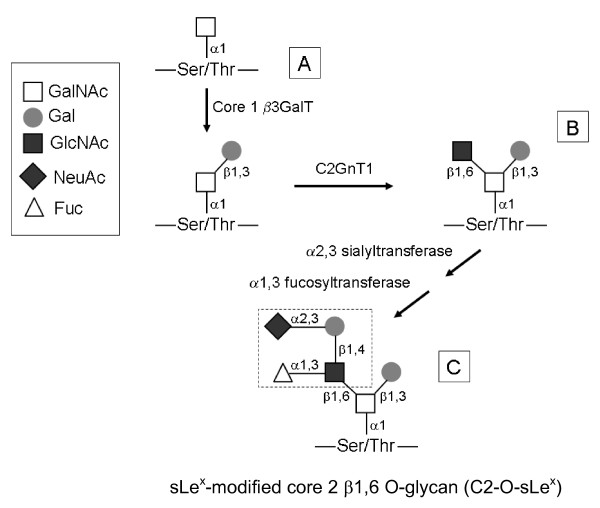
Figure 1.
Diagram of core 2 β1,6 O-glycan synthesis. (A) Core 1 O-glycans are synthesized by addition of β1,3 galactose to N-acetylgalactosamine. (B) The C2GnT1 enzyme converts an unsubstituted core 1 O-glycan to a core 2 β1,6 O-glycan. (C) Core 2 can be further modified by α2,3 sialyltransferase and α1,3 fucosyltransferase, forming a sLex terminus (dotted box). These modifications result in the synthesis of the sLex-modified core 2 β1,6 O-glycan (C2-O-sLex) structure. The figure is simplified and some enzymatic steps are omitted for clarity. GalNAc, N-acetylgalactosamine; Gal, galactose; GlcNAc, N-acetylglucosamine; NeuAc, sialic acid; Fuc, fucose.