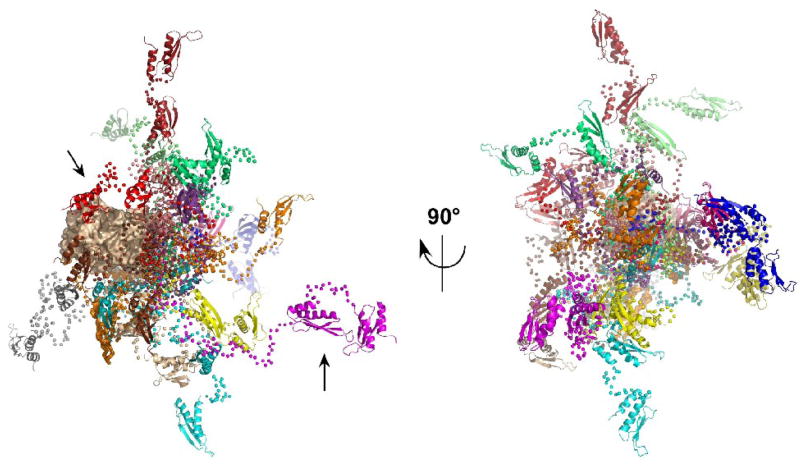
Figure 4.
Overlay of PKR structures selected by EOM. The 20 PKR conformations from the ensemble selected by EOM analysis of the 2 mg/mL SAXS data are superimposed by alignment on the kinase domain. The kinase domain is shown in a tan surface representation, the dsRBM1 and dsRBM2 are shown in ribbon representation and the Cα atoms in the flexible linkers are shown as spheres. Each of the 20 conformations is depicted in a different color. In the view on the left, the kinase domain is oriented with the C-lobe on the left and the N-lobe to the right. The arrows indicate representative compact (red) and extended (magenta) conformers. The ensemble on the right is rotated by −90° about the y-axis such that the kinase N-lobe points out of the page.