Abstract
Industrial enzymes are often immobilized via chemical cross-linking onto solid supports to enhance stability and facilitate repeated use in bioreactors. For starch-degrading enzymes, immobilization usually places constraints on enzymatic conversion due to the limited diffusion of the macromolecular substrate through available supports. This study describes the one-step immobilization of a highly thermostable α-amylase (BLA) from Bacillus licheniformis and its functional display on the surface of polyester beads inside engineered Escherichia coli. An optimized BLA variant (Termamyl) was N-terminally fused to the polyester granule-forming enzyme PhaC of Cupriavidus necator. The fusion protein lacking the signal sequence mediated formation of stable polyester beads exhibiting α-amylase activity. The α-amylase beads were assessed with respect to α-amylase activity, which was demonstrated qualitatively and quantitatively. The immobilized α-amylase showed Michaelis-Menten enzyme kinetics exerting a Vmax of about 506 mU/mg of bead protein with a Km of about 5 μM, consistent with that of free α-amylase. The stability of the enzyme at 85°C and the capacity for repeated usage in a starch liquefaction process were also demonstrated. In addition, structural integrity and functionality of the beads at extremes of pH and temperature, demonstrating their suitability for industrial use, were confirmed by electron microscopy and protein/enzyme analysis. This study proposes a novel, cost-effective method for the production of immobilized α-amylase in a single step by using the polyester granules forming protein PhaC as a fusion partner in engineered E. coli.
α-Amylases (EC 3.2.1.1) are among the most widely used technological enzymes, with applications in food processing, detergent manufacture, and several other industries (5, 13, 25) that use starch liquefaction (21). While they are widely distributed in plants, animals, and microorganisms, the secreted α-amylase from Bacillus licheniformis (BLA) is the one most extensively used in the starch industry (5), due mainly to its thermostability (7, 13). However, BLA has been subjected to protein engineering approaches in order to enhance its technically relevant enzyme properties such as greater temperature stability and a wider pH activity range (16, 24).
Industrial enzymes are frequently immobilized onto solid supports in order to increase resistance to fluctuations in conditions such as pH and temperature (18) and to facilitate repeated usage. Although BLA has previously been successfully immobilized (25, 27, 28), it has been suggested that access to large starch molecules through pores in typical supports can be a limitation (27). Therefore, a system that allows free, high-density contact between the immobilized enzyme and its substrate would be an advantage. However, covalent immobilization of BLA as well as any other biocatalyst requires a laborious and costly production process involving production of the enzyme, generation of supports, and chemical cross-linking of both components. Here, the optimized BLA (Termamyl) was further engineered to mediate intracellular formation of spherical polyester beads that display highly active α-amylase covalently attached at high density and homogenous functional orientation.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
Bacterial strains used in this study are listed in Table 1. All Escherichia coli strains were grown at 37°C unless otherwise stated. When required, the following antibiotics were added (concentration): ampicillin (75 μg/ml), chloramphenicol (50 μg/ml), tetracycline (12.5 μg/ml), and kanamycin (12.5 μg/ml). For polyester bead production, cells were grown at 37°C to an optical density at 600 nm of 0.45 and then induced with the addition of 1 mM IPTG (isopropyl-β-d-thiogalactopyranoside). Following induction, cultures were cultivated at 30°C with shaking for 44 h.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Description | Source or reference |
|---|---|---|
| E. coli strains | ||
| Top 10 | F−mcrA Δ(mrr-hsdRMS-mcrBC) φ80lacZΔM15 ΔlacX74 recA1 ara139 Δ(ara-leu)7697 galU galK rpsL(Strr) endA1 nupG | Invitrogen |
| Origami B (DE3) | F−ompT hsdSB (rB− mB−) gal dcm lacY1 ahpC (DE3) gor522::Tn10trxB (Kanr Tetr) | Novagen |
| BL21(DE3) | F−ompT hsdSB (rB− mB−) gal dcm (DE3) | Novagen |
| Plasmids | ||
| pMCS69 | pBBR1MCS derivative containing phaA and phaB genes from C. necator | 2 |
| pET14b | Apr; T7 promoter | Novagen |
| pETC | Plasmid coding for PhaC wild type under the control of T7 promoter | 20 |
| pET14b-SAphaC | Plasmid coding for streptavidin(SA)-PhaC fusion under the control of T7 promoter | 20 |
| pET 14b-BLAphaC | Plasmid coding for BLA(+ss)PhaC fusion under the control of T7 promoter | This study |
| pET14b-BLA(−ss)phaC | Plasmid coding for BLA(−ss)PhaC fusion under the control of T7 promoter | This study |
Plasmids and oligonucleotides.
Plasmids used in this study are listed in Table 1. General cloning procedures and DNA isolation were performed as described elsewhere (22). DNA primers, deoxynucleoside triphosphate, T4 DNA ligase, and Taq polymerase were purchased from Invitrogen (CA). The plasmid pJ204BLA was synthesized by GeneScript Corp. Chemical reagents were purchased from Sigma Aldrich (St. Louis, MO).
Construction of plasmids for production of BLA-displaying polyester beads.
The DNA sequence encoding the stabilized variant of full-length BLA (α-amylase) (16) from B. licheniformis was obtained codon optimized for expression in E. coli from GenScript Corp. as an insert within the plasmid pJ204. To enable production of a BLA-PhaC fusion protein, the full-length BLA-encoding gene was initially cloned into the SpeI site of plasmid pET14b-SAphaC (20), replacing the existing insert. The resulting plasmid, pET14b-BLAphaC, was used to transform E. coli BL21(DE3) competent cells harboring the plasmid pMCS69, which mediates the synthesis of the precursor R-3-hydroxybutyryl-coenzyme A (CoA) required for polyester synthesis and, hence, polyester bead formation (1, 2). To assess the role of the signal sequence, primers BLA-Nterm-Leader (5′-GTGGGGGACTAGTATGGCCGCTAACCTGAAC-GGTACCCTGATGCAG- 3′) and BLA-Cterm (5′-AAAGAGACTAGTGCCACCACC-ACCACCGCGCTGGACGTAGATGG-3′) were used to amplify the BLA gene encoding BLA minus the signal sequence from pJ204BLA. The PCR product was cloned into the SpeI restriction site of the plasmid pET14b-BLAphaC after removal of the original BLA-encoding gene region, producing pET14b-BLA(−ss)phaC (where −ss indicates the absence of the signal sequence), which was then used to transform E. coli BL21(DE3) and Origami B (DE3) strains harboring pMCS69. The DNA sequence of the new plasmid construct was confirmed by DNA sequencing.
To produce control beads that do not display α-amylase activity, the previously described plasmid pETC (20), which encodes only the wild-type PhaC of Cupriavidus necator, was used in E. coli Origami B (DE3) in the presence of plasmid pMCS69.
Protein analysis.
Bead protein profiles were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) as described elsewhere (12). Gels were stained with Coomassie brilliant blue G250. Protein concentrations were determined using the Bradford protein quantification method (3).
MALDI-TOF MS.
In order to identify the BLA-PhaC fusion protein, the band of interest was cut out from the gel and subjected to matrix-assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF MS). Peptides produced by tryptic digestion were analyzed as previously described (6).
Polyester analysis.
Production of the polyester polyhydroxybutyrate, which indicated polyester bead formation, was determined qualitatively and quantitatively by gas chromatography(GC)/MS (4).
Polyester bead isolation.
Polyester beads were isolated from recombinant E. coli cells using mechanical cell disruption and ultracentrifugation on a glycerol gradient as previously described (10). Control beads were produced from E. coli Origami B (DE3) cells harboring pMCS69 and pETC.
Microscopy.
Polyester granules produced inside bacterial cells were visualized with fluorescence microscopy following Nile red staining, as previously described (19). Isolated beads were prepared for transmission electron microscopy (TEM) and viewed with a Philips CM10 microscope as previously described (6).
TEM of beads subjected to extreme conditions of temperature and pH was obtained by washing and resuspending the beads in 20 mM Tris HCl (pH 8.0) and by incubation at various temperatures or pH values. Following incubation, the beads were sedimented by centrifugation (5,000 × g for 15 min at 4°C), washed twice with 50 mM potassium phosphate buffer, pH 7.5, and analyzed by TEM.
Assessment of α-amylase activity of the BLA beads.
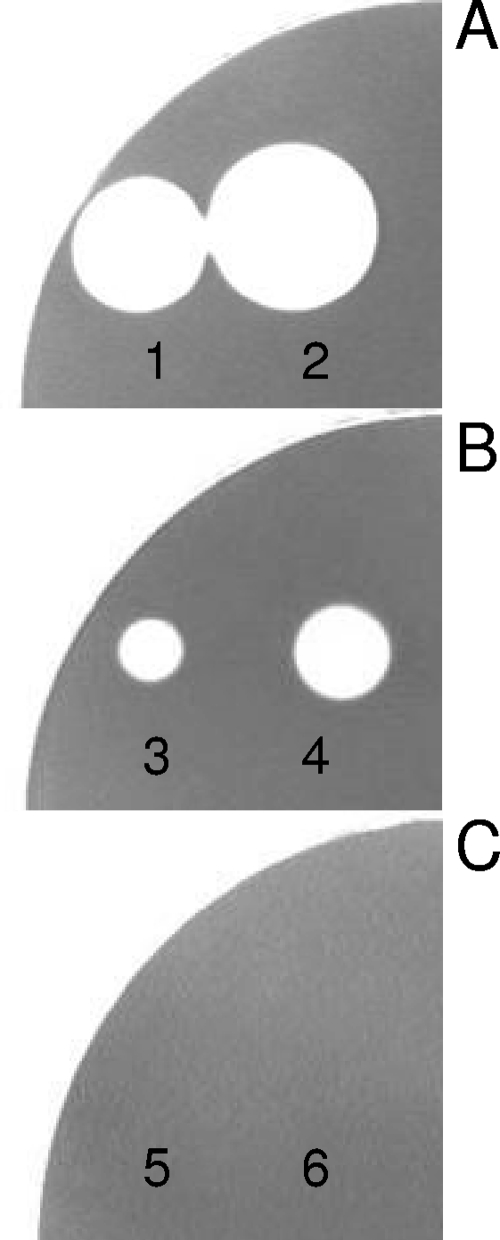
To qualitatively assess the ability of engineered beads to hydrolyze starch, starch agar was prepared using LB medium supplemented with 0.5% (wt/vol) tryptone, 0.3% (wt/vol) casein, 0.5% (wt/vol) NaCl, and 2.5% (wt/vol) potato starch. The enzyme α-amylase from B. licheniformis (Sigma A3403) was included as positive control for starch-degrading enzyme activity (see Fig. 2A). Control beads were used as a negative control (see Fig. 2C). The BLA beads and control beads, as well as the free α-amylase enzyme, were incubated at 37°C overnight on the surface of starch agar plates. At the end of incubation, the plates were washed with distilled water and stained with 10 ml of Lugol's iodine solution (8).
FIG. 2.
Qualitative analysis of BLA (α-amylase) activity using starch agar and Lugol's iodine staining. Sectors are as follows: A, α-amylase (Sigma 3403); B, BLA-PhaC beads; C, control PhaC beads. Protein amounts for the numbered positions are as follows: 1, 56 μg; 2, 180 μg; 3 and 5, 21 μg; 4 and 6, 70 μg.
The enzymatic activity of the engineered beads was quantitatively assessed using a 3,5-dinitrosalicyclic acid colorimetric assay for the α-amylase (EC 3.2.1.1; Sigma A3403) (29). Starch was prepared for all experiments in the buffer of desired pH and gelatinized by heating until boiling for 10 min. BLA beads for all experiments were prepared as buffer suspensions containing 500 μg of protein/ml. All measurements were conducted in triplicate.
Effect of temperature on enzymatic activity.
To determine the effect of temperature on activity, starch and beads prepared in 20 mM Tris HCl, pH 8.0, were incubated at temperatures ranging from 35°C to 85°C in a shaking water bath for 2.5 h. Samples were removed from the reaction mixtures at half-hourly intervals and sedimented by centrifugation (5,000 × g for 5 min). The supernatant was assessed for the presence of maltose.
Effect of pH on enzymatic activity.
To determine the effect of pH on activity, the beads and starch were incubated at 75°C with shaking in the following 20 mM buffers: sodium acetate (pH 4, 5, and 5.6), Tris-HCl (pH 7 and 8), sodium borate (pH 9 and 10), and sodium carbonate/bicarbonate (pH 11). Samples were removed from the reaction mixtures at half-hourly intervals and sedimented by centrifugation (5,000 × g for 5 min). The supernatant was assessed for the presence of maltose.
Temperature stability.
To assess stability at high temperatures, the beads were preincubated without starch at 85°C and 95°C in 20 mM Tris-HCl, pH 8.0, for 5 h with shaking. Samples were removed at hourly intervals and used for the starch hydrolysis assay as described above, using 5 mg/ml starch in 20 mM Tris-HCl, pH 8.0, at 75°C.
Immobilized BLA kinetics.
To determine the kinetics of immobilized BLA, starch at concentrations ranging 1.25 to 25 mg/ml was incubated with 500 μg/ml bead suspensions at 55°C in a shaking water bath for 2.5 h. Samples were removed from the reaction mixture at half-hourly intervals and sedimented by centrifugation (5,000 × g for 5 min). The supernatant was assessed for the presence of maltose.
Reusability.
To assess the effect of repeated usage on enzymatic activity, the assay was conducted under optimum conditions. At the end of 2.5 h, the remaining starch-bead mixture was sedimented by centrifugation (5,000 × g at 4°C for 15 min). The beads were washed three times in 20 mM Tris-HCl, pH 8.0, and then resuspended to the original concentration, and the assay was repeated under identical conditions.
RESULTS
The BLA-PhaC fusion protein lacking the signal sequence mediates production of polyester beads.
The plasmid pET 14b-BLAphaC encoding the full-length BLA gene did not produce transformants in the E. coli Bl21(DE3) and Origami B (DE3) strains. This plasmid could be propagated only in E. coli strains not enabling T7 promoter-dependent gene expression. However, plasmid pET14b-BLA(−ss)phaC containing the hybrid gene encoding BLA lacking the signal sequence required for secretion and translationally fused to the N terminus of PhaC mediated overproduction of the respective BLA-PhaC fusion protein in both E. coli strains [for Origami B (DE3) cells, see Fig. 1]. The fusion protein was demonstrated by SDS-PAGE analysis of whole-cell lysates of induced E. coli Origami B (DE3) harboring only plasmid pET14b-BLA(−ss)phaC (data not shown) as well as both plasmids pET14b-BLA(−ss)phaC and pMCS69 (Fig. 1). Plasmid pMCS69 contains the genes phaA (β-ketothiolase) and phaB (acetoacetyl-CoA reductase) from C. necator that mediate provision of the polyester precursor (R)-3-hydroxybutyryl-CoA. GC/MS analysis showed that pET14b-BLA(−ss)phaC mediated production of the polyester polyhydroxybutyrate in the presence of plasmid pMCS69 to a level comparable to the wild-type PhaC encoded by pETC (data not shown). Since E. coli Origami B (DE3) showed 20% more polyhydroxyalkanoate accumulation than E. coli BL21(DE3), it was selected as the production host for subsequent bead production.
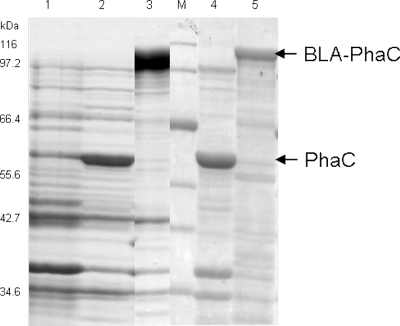
FIG. 1.
Overproduction of the BLA-PhaC fusion in E. coli Origami B (DE3) cells demonstrated by SDS-PAGE analysis. Whole cells (lanes 1 to 3) harboring various plasmids and, if applicable, isolated polyester beads (lanes 4 and 5) were analyzed. Lane 1, pET14b (vector control); lane 2, pETC (wild-type control); lane 3, pET14b-BLA(−ss)phaC; M, molecular mass standard; lane 4, pETC (wild-type control); lane 5, pET14b-BLA(−ss)phaC. Arrows indicate the BLA-PhaC fusion protein and the wild-type PhaC protein.
BLA-PhaC is abundantly attached to polyester granules.
The BLA-PhaC fusion protein was shown by SDS-PAGE to be overproduced at the granule surface (Fig. 1). No free fusion protein could be detected in the cell-free supernatant or in the soluble cytosolic fraction (data not shown). The identity of the fusion proteins in whole-cell lysates and attached to granules was confirmed by tryptic peptide fingerprinting combined with MALDI-TOF MS analysis (see Table S1 in the supplemental material). No fusion protein was produced in the induced whole cells harboring the control vectors pET14b or pETC together with pMCS69. In the latter cells, wild-type PhaC could be detected (Fig. 1). The purity of isolated polyester beads was confirmed by determining the polyester content by GC/MS. A high polyester content reflects efficient removal of cellular components.
BLA beads show α-amylase activity.
To qualitatively assess α-amylase activity, hydrolysis of starch by the BLA beads and the commercial α-amylase was analyzed on starch agar (Fig. 2). Following incubation and staining with Lugol's iodine solution, α-amylase activity was observed for the commercial enzyme (positive control) and the BLA beads, as indicated by a clearing in the starch agar. No activity was detected in case of the PhaC beads (negative control) (Fig. 2).
Enzymatic characterization of α-amylase immobilized to the polyester beads.
To characterize the immobilized α-amylase, enzyme kinetic studies as well as reusability and temperature stability studies were conducted. Beads that were exposed to extreme conditions such as high temperature (85°C and 95°C) or extreme pH values (pH 4 and 11) were assessed for structural integrity and attachment of the fusion protein by TEM and SDS-PAGE, respectively (see Fig. S1 in the supplemental material).
Effect of temperature on enzymatic activity of BLA beads.
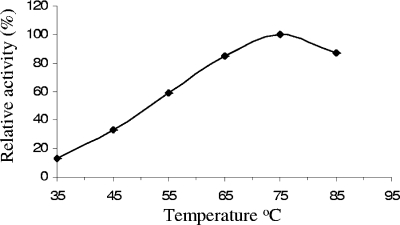
The rate of the conversion of starch to maltose increased steadily with the incubation temperature up to 75°C and declined at 85°C (Fig. 3). Therefore, 75°C was considered as the optimum temperature for enzyme activity of immobilized BLA.
FIG. 3.
Temperature versus relative activity profile of immobilized BLA (BLA-PhaC beads). Standard deviation values were <0.024%. A relative α-amylase activity of 100% corresponds to 506 mU/mg of bead protein.
Effect of pH on enzymatic activity of BLA beads.
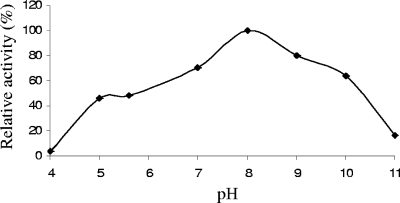
The rate of conversion of starch to maltose was highest at pH 8.0 (Fig. 4). Enzyme activity was low at extreme pH values (pH 4 and 11). Thus, pH 8.0 was considered an optimum pH value for the activity of immobilized α-amylase.
FIG. 4.
pH versus relative activity profile of immobilized BLA (BLA-PhaC beads). Standard deviation values were <0.016%. A relative α-amylase activity of 100% corresponds to 506 mU/mg of bead protein.
Kinetic analysis of immobilized BLA.
The highest relative activity was obtained at a starch concentration of 5 mg/ml. The total activity was 313.2 U from a 1-liter culture.
Determination of kinetic parameters of BLA immobilized at the bead surface.
The BLA activity of isolated BLA beads was monitored for 2.5 h (data not shown) at starch concentrations from 0 μM to 75 μM. The correlation of the voided volume with the substrate concentration could be fitted to Michaelis-Menten kinetics with the aid of nonlinear regression analysis. A Km of about 5 μM and a Vmax of about 506 mU/mg of bead protein could be derived.
Reusability of BLA beads.
Reusability of the beads was assessed by using the initial assay followed by two successive processing cycles using the same BLA beads. While an initial decline of 28% activity was observed in the second process cycle, this level of activity was maintained in the third cycle, with 78% of initial activity (data not shown).
Temperature stability of BLA beads.
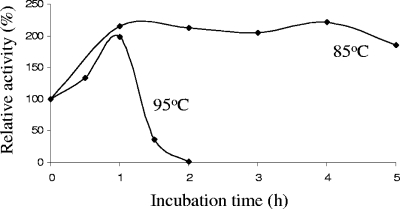
BLA beads were incubated at 85°C and 95°C, and the α-amylase activity of BLA beads was assessed as a function of time. The α-amylase activity of the beads was unaffected after incubation at 85°C for 5 h, but at 95°C a significant decline was observed after 1 h (Fig. 5).
FIG. 5.
Temperature stability of immobilized BLA (BLA-PhaC beads). Standard deviation values were <0.019. A relative α-amylase activity of 100% corresponds to 506 mU/mg of bead protein.
Structural integrity of BLA beads and stability of the natural cross-link immobilizing BLA to the beads.
BLA beads were exposed for 2.5 h to extreme pH values (pH 4 and 11) at 75°C and high temperatures (85°C and 95°C) at pH 8.0. TEM analysis of these beads indicated that the shape and size of the beads were not affected (see Fig. S1 in the supplemental material).
DISCUSSION
Immobilization of α-amylases for continuous industrial use has mainly involved covalent attachment to various supports including glass beads (11), polymeric microspheres (28), cellulose beads (25), and reactive membranes and fibers (27, 28). In contrast to current immobilization methods, which involve several stages, this study describes the use of engineered E. coli to produce a highly thermostable BLA variant on the surface of polyester beads in a single step. A BLA variant harboring five mutations that enhanced thermostability (16) was successfully displayed on the polymer bead surface under the control of the strong T7 promoter (Fig. 1 and 2).
E. coli is widely used as an expression host for the production of industrially relevant amylases (14, 15, 26, 29). In most cases, the enzyme is produced extracellularly and recovered from the cultivation medium or periplasmic space (26, 29). While some reports have described the intracellular production of α-amylase in E. coli (17, 23), extracellular production is generally preferred in the industrial context to facilitate correct protein folding and easier downstream processing (29). Thus, the direct immobilization and easy purification of the enzyme reported here were considered as industrially advantageous.
In this study, the translational fusion of the BLA C terminus to PhaC initially attempted using the full-length BLA sequence including its signal peptide was unsuccessful. Transfer of this plasmid was achieved only into E. coli strains lacking the T7 RNA polymerase gene, which suggested that production of the signal peptide comprising the fusion protein might have a toxic effect on cells. This was consistent with a recent study (17) and was further supported here by construction of a hybrid gene encoding the BLA-PhaC fusion without the signal peptide, which resulted in T7 promoter-based fusion protein overproduction with simultaneous polyester bead formation. Hence, the signal peptide is not required for proper folding of the α-amylase, which is consistent with a previous study by Lo et al. (15) (Fig. 1). Whole-cell protein analysis suggested strong overproduction of the fusion protein, i.e., very efficient bead production inside the cells as reflected by only a little gain in purity when the total protein of isolated beads was assessed.
An optimum temperature range from 55 to 70°C and an optimum pH of 5.2 were recently reported for an immobilized α-amylase and compared to optimum values of 55°C and pH 5.6 for free BLA (25). For the BLA beads, the optimum temperature of 75°C (Fig. 3), an optimum pH of 8 (Fig. 4), and stability at 85°C and 95°C (Fig. 5) suggested suitability as an additive in laundry detergents (9) and superior enzyme properties compared to free BLA (13).
The immobilized BLA indicated a Michalis-Menten-type kinetics, with a Km of 5 μM, suggesting a high binding affinity compared with the Km values of 9.6 μM and 44 μM for free BLA and BLA chemically cross-linked to cellulose beads, respectively (25).
TEM analyses showed structural integrity of the BLA beads under extreme conditions of temperature and pH while SDS-PAGE indicated that the BLA-PhaC fusion remained attached to the beads under extreme conditions, which suggested a stable bond between the fusion protein and the polyester core. With regard to reusability, the maintenance of high enzyme activity in the second and third process cycle demonstrated that the beads may be reused, an important property of immobilized enzymes (25, 27).
Supplementary Material
Acknowledgments
This study was supported by research grants from Massey University and the International Investment Opportunity Fund from the Foundation of Science, Research and Technology in New Zealand.
We gratefully acknowledge the design of oligonucleotides and the construction of plasmid pET14b-BLAphaC by U. Remminghorst (Massey University). We are also indebted to M. Showell (Procter & Gamble) for helpful scientific discussions.
Footnotes
Published ahead of print on 5 February 2009.
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1.Amara, A. A., and B. H. A. Rehm. 2003. Replacement of the catalytic nucleophile cysteine-296 by serine in class II polyhydroxyalkanoate synthase from Pseudomonas aeruginosa-mediated synthesis of a new polyester: identification of catalytic residues. Biochem. J. 374:413-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amara, A. A., A. Steinbuchel, and B. H. A. Rehm. 2002. In vivo evolution of the Aeromonas punctata polyhydroxyalkanoate (PHA) synthase: isolation and characterization of modified PHA synthases with enhanced activity. Appl. Microbiol. Biotechnol. 59:477-482. [DOI] [PubMed] [Google Scholar]
- 3.Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 4.Brandl, H., R. A. Gross, R. W. Lenz, and R. C. Fuller. 1988. Pseudomonas oleovorans as a source of poly(β-hydroxyalkanoates) for potential applications as biodegradable polyesters. Appl. Environ. Microbiol. 54:1977-1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bravo Rodriguez, V., E. J. Alameda, J. F. M. Gallegos, A. R. Requena, and A. I. G. Lopez. 2006. Enzymatic hydrolysis of soluble starch with an alpha-amylase from Bacillus licheniformis. Biotechnol. Prog. 22:718-722. [DOI] [PubMed] [Google Scholar]
- 6.Grage, K., and B. H. A. Rehm. 2008. In vivo production of scFv-displaying biopolymer beads using a self-assembly-promoting fusion partner. Bioconjug. Chem. 19:254-262. [DOI] [PubMed] [Google Scholar]
- 7.Gray, G. L., S. E. Mainzer, M. W. Rey, M. H. Lamsa, K. L. Kindle, C. Carmona, and C. Requadt. 1986. Structural genes encoding the thermophilic α-amylases of Bacillus stearothermophilus and Bacillus licheniformis. J. Bacteriol. 166:635-643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Humason, G. L. 1972. Animal tissue techniques, 3rd ed. W. H. Freeman and Co., San Francisco, CA.
- 9.Ito, S., T. Kobayashi, Y. Hatada, and K. Horikoshi. 2005. Enzymes in modern detergents, p. 151-164. In J. L. Barredo (ed.), Microbial enzymes and biotransformations. Humana Press, Inc., Totowa, NJ.
- 10.Jahns, A. C., R. G. Haverkamp, and B. H. A. Rehm. 2008. Multifunctional inorganic-binding beads self-assembled inside engineered bacteria. Bioconjug. Chem. 19:2072-2080. [DOI] [PubMed] [Google Scholar]
- 11.Kahraman, M. V., G. Bayramoglu, N. Kayaman-Apohan, and A. Gungor. 2007. α-Amylase immobilization on functionalized glass beads by covalent attachment. Food Chem. 104:1385-1392. [Google Scholar]
- 12.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 13.Lee, S., H. Oneda, M. Minoda, A. Tanaka, and K. Inouye. 2006. Comparison of starch hydrolysis activity and thermal stability of two Bacillus licheniformis α-amylases and insights into engineering alpha-amylase variants active under acidic conditions. J. Biochem. 139:997-1005. [DOI] [PubMed] [Google Scholar]
- 14.Lin, L. L., and W. H. Hsu. 1997. Lactose-induced expression of Bacillus sp. TS-23 amylase gene in Escherichia coli regulated by a T7 promoter. Lett. Appl. Microbiol. 24:365-368. [DOI] [PubMed] [Google Scholar]
- 15.Lo, H. F., L. L. Lin, C. C. Li, W. H. Hsu, and C. T. Chang. 2001. The N-terminal signal sequence and the last 98 amino acids are not essential for the secretion of Bacillus sp. TS-23 α-amylase in Escherichia coli. Curr. Microbiol. 43:170-175. [DOI] [PubMed] [Google Scholar]
- 16.Machius, M., N. Declerck, R. Huber, and G. Wiegand. 2003. Kinetic stabilization of Bacillus licheniformis α-amylase through introduction of hydrophobic residues at the surface. J. Biol. Chem. 278:11546-11553. [DOI] [PubMed] [Google Scholar]
- 17.Murakami, S., H. Nishimoto, Y. Toyama, E. Shimamoto, S. Takenaka, J. Kaulpiboon, M. Prousoontorn, T. Limpaseni, P. Pongsawasdi, and K. Aoki. 2007. Purification and characterization of two alkaline, thermotolerant α-amylases from Bacillus halodurans 38C-2-1 and expression of the cloned gene in Escherichia coli. Biosci. Biotechnol. Biochem. 71:2393-2401. [DOI] [PubMed] [Google Scholar]
- 18.Novick, S. J., and J. D. Rozzell. 2005. Immobilization of enzymes by covalent attachment, p. 247-272. In J. L. Barredo (ed.), Microbial enzymes and biotransformations. Humana Press, Totowa, NJ.
- 19.Peters, V., and B. H. A. Rehm. 2005. In vivo monitoring of PHA granule formation using GFP-labeled PHA synthases. FEMS Microbiol. Lett. 248:93-100. [DOI] [PubMed] [Google Scholar]
- 20.Peters, V., and B. H. A. Rehm. 2008. Protein engineering of streptavidin for in vivo assembly of streptavidin beads. J. Biotechnol. 134:266-274. [DOI] [PubMed] [Google Scholar]
- 21.Rivera, M. H., A. Lopez-Munguia, X. Soberon, and G. Saab-Rincon. 2003. α-Amylase from Bacillus licheniformis mutants near to the catalytic site: effects on hydrolytic and transglycosylation activity. Protein Eng. 16:505-514. [DOI] [PubMed] [Google Scholar]
- 22.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 23.Shahhoseini, M., A. A. Ziaee, and N. Ghaemi. 2003. Expression and secretion of an alpha-amylase gene from a native strain of Bacillus licheniformis in Escherichia coli by T7 promoter and putative signal peptide of the gene. J. Appl. Microbiol. 95:1250-1254. [DOI] [PubMed] [Google Scholar]
- 24.Shaw, A., R. Bott, and A. G. Day. 1999. Protein engineering of alpha-amylase for low pH performance. Curr. Opin. Biotechnol. 10:349-352. [DOI] [PubMed] [Google Scholar]
- 25.Shewale, S. D., and A. B. Pandit. 2007. Hydrolysis of soluble starch using Bacillus licheniformis α-amylase immobilized on superporous CELBEADS. Carbohydr. Res. 342:997-1008. [DOI] [PubMed] [Google Scholar]
- 26.Shiina, S., T. Ohshima, and M. Sato. 2007. Extracellular production of α-amylase during fed-batch cultivation of recombinant Escherichia coli using pulsed electric field. J. Electrostat. 65:30-36. [Google Scholar]
- 27.Tanyolac, D., B. I. Yuruksoy, and A. R. Ozdural. 1998. Immobilization of a thermostable α-amylase, termamyl, onto nitrocellulose membrane by Cibacron Blue F3GA dye binding. Biochem. Eng. J. 2:179-186. [Google Scholar]
- 28.Tumturk, H., S. Aksoy, and N. Hasirci. 2000. Covalent immobilization of α-amylase onto poly(2-hydroxyethyl methacrylate) and poly(styrene-2-hydroxyethyl methacrylate) microspheres and the effect of Ca2+ ions on the enzyme activity. Food Chem. 68:259-266. [Google Scholar]
- 29.Yamabhai, M., S. Emrat, S. Sukasem, P. Pesatcha, N. Jaruseranee, and B. Buranabanyat. 2008. Secretion of recombinant Bacillus hydrolytic enzymes using Escherichia coli expression systems. J. Biotechnol. 133:50-57. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.