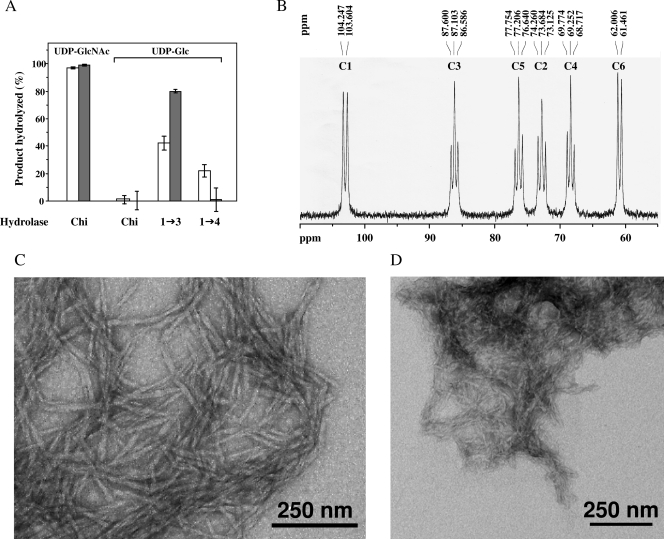
FIG. 2.
Analysis of the polysaccharides synthesized in vitro by membrane fractions. (A) Polysaccharides synthesized by plasma membranes (□) or DRMs (░⃞) in the presence of either UDP-N-acetyl-[14C]glucosamine (UDP-GlcNAc) or UDP-[14C]glucose (UDP-Glc) were hydrolyzed by specific glycoside hydrolases. Chi, chitinase; 1→3, (1→3)-β-d-glucanase; 1→4, cellulase mixture. The percentage of hydrolysis was determined by liquid scintillation counting as described in Materials and Methods. (B) 13C-NMR analysis in (CD3)2SO of the polysaccharides synthesized by DRMs in the presence of 13C-enriched UDP-glucose. The spectrum is characteristic of a linear (1→3)-β-d-glucan. (C) Transmission electron micrograph of the (1→3)-β-d-glucan synthesized in vitro by DRMs incubated in the presence of UDP-glucose. The sample was negatively stained with 4% uranyl acetate. (D) Same as in panel C, but in the case of chitin synthesized in vitro in the presence of UDP-GlcNAc.