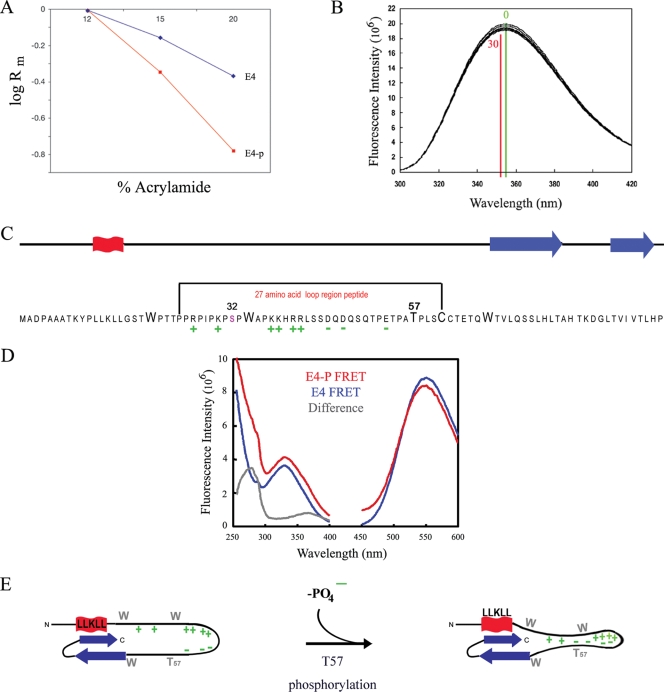
FIG. 4.
T57 phosphorylation triggers 16E1^E4 structural change. (A) Dependence of electrophoretic mobilities (Rm) of the 16E1^E4 phosphorylated (E4-p) and unphosphorylated (E4) species on the acrylamide concentration in SDS-PAGE (Ferguson plot). (B) Intrinsic fluorescence upon the phosphorylation of His-16E1^E4 by ERK was monitored. The fluorescence maxima prior to phosphorylation (time, 0 min; green) and upon the completion of phosphorylation (time, 30 min; red) are indicated on the plot. The decrease in fluorescence intensity and the red shift of the spectrum accompanying phosphorylation are consistent with the tryptophan residues becoming more buried in a hydrophobic environment. (C) The 16E1^E4 protein has elements of secondary structure at its C and N termini (red, α helix; blue, β sheet). T57 is located in a highly charged unstructured region of the protein, and its phosphorylation contributes to the polarization of this region. The effect of T57 phosphorylation on this unstructured region of the protein was assessed using a 27-amino-acid peptide as indicated. (D) The effect of T57 phosphorylation on the structure of the peptide (indicated in panel C) was monitored by recording fluorescent resonance energy transfer (FRET) between the tryptophan residue and a fluorescence acceptor molecule on the terminal cysteine. The excitation (250- to 400-nm) and emission (450- to 600-nm) spectra of the phosphorylated (E4-P) and unphosphorylated (E4) peptides are shown. The peak at 280 nm in the difference spectrum reveals that fluorescence transfer is observed only in the phosphorylated peptide, which indicates that T57 phosphorylation compacts the peptide. (E) Schematic summarizing the effect of T57 phosphorylation by ERK on the structure of 16E1^E4. Upon phosphorylation, the addition of a negative charge on T57 increases the charge polarization in the loop region of the protein and, as indicated by the findings of the tryptophan fluorescence studies, results in additional restraints in this region.