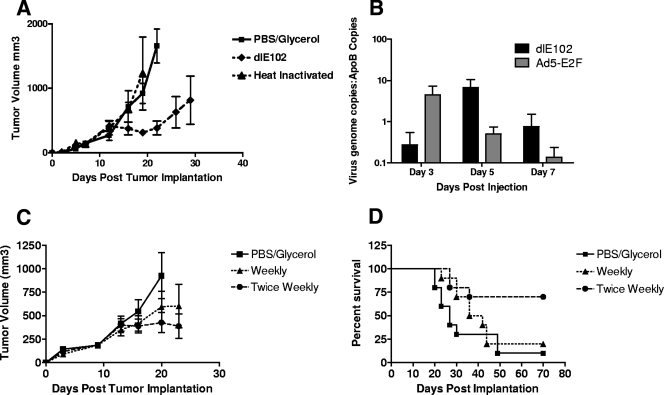
FIG. 2.
In vivo efficacy of dlE102 in an immunocompetent syngeneic tumor model. (A) Female BALB/c mice bearing subcutaneous CT26 tumors were injected i.t. when the average tumor size reached approximately 150 mm3 with the vehicle (PBS-10% [vol/vol] glycerol), dlE102 (1 × 107 PFU in a 50-μl volume), or heat-inactivated dlE102 (50 μg of total protein in a 50-μl volume) on days 8, 10, and 15 post tumor implantation for a total of three injections. Tumor volume was determined by caliper measurement and expressed as the mean tumor volume (cubic millimeters plus the standard error of the mean; n = 5 per group). (B) The level of virus replication within the tumor was determined in female BALB/c mice bearing CT26 tumors with a single i.t. injection of 1 × 107 PFU dlE102 or Ad5-E2F. Mice were euthanized 3, 5, and 7 days post virus injection, tumors were homogenized, the genomic and viral DNAs were extracted, and the number of adenovirus genome copies in the liver was determined by qPCR. The data are presented as the number of virus genome copies per copy of cellular ApoB genome to normalize for total genomic DNA content. (C and D) The effect of repeat administration of the dlE102 vector was determined in the CT26 tumor model. Female BALB/c mice bearing subcutaneous CT26 tumors were injected i.t. with the vehicle (PBS-10% [vol/vol] glycerol) twice weekly or either a weekly dose or a twice-weekly dose of dlE102 (1 × 107 PFU in a 50-μl total volume). The tumor volume (panel C; cubic millimeters plus the standard error of the mean; n = 10 per group) and overall survival (panel D) were determined.