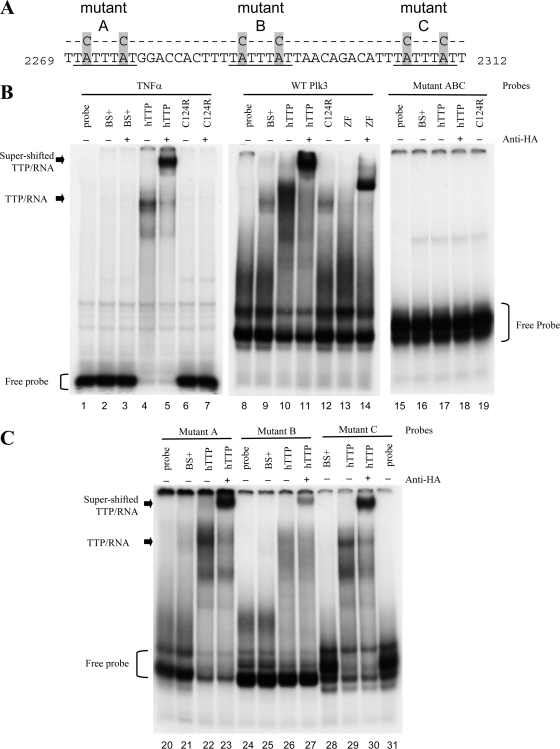
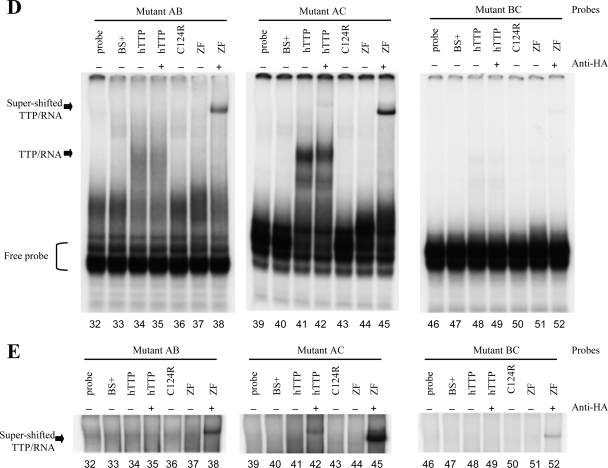
FIG. 4.
RNA gel shifts of WT and mutant Plk3 AREs with TTP. (A) The sequences of the RNA probes used are shown and include the three potential TTP binding sites within bp 2269 to 2312 of GenBank accession number NM_013807.2. The three core heptamers of the prospective TTP binding sites are underlined. The locations of the A-to-C mutations within each heptamer are highlighted. (B to E) RNA gel shift autoradiographs. The indicated RNA probes were incubated with cytosolic extracts derived from HEK293 cells transfected with either vector alone [pBS(+)], WT human TTP plasmid (hTTP), the mutant TTP plasmid with the C124R zinc finger mutation (C124R), or a fragment of TTP containing the tandem zinc finger domains (ZF). Cell extracts were incubated with (+) or without (−) anti-HA antibodies prior to the addition of RNA probes. Lanes 1 to 7 represent conditions under which the TNF ARE probe, used as a positive control, was incubated without (lane 1) or with (lanes 2 to 7) cytosolic extracts. Lanes 8 to 14 represent conditions under which the WT ARE of Plk3 was incubated without (lane 8) or with (lanes 9 to 14) cytosolic extracts. Only the presence of either unmutated hTTP or the zinc finger domains alone caused a shift of both TNF and WT Plk3 probes. Lanes 15 to 19 represent conditions in which the ABC mutant Plk3 probe was incubated without (lane 15) or with (lanes 16 to 19) the same cytosolic extracts. The unbound probes are denoted with brackets, and the complexes formed with hTTP are indicated with arrows. The supershifted TTP/RNA complex was observed when anti-HA monoclonal antibody was incubated with the indicated cell extracts for 10 min prior to the addition of probe. (C) Plk3 RNA probes with mutations in first (mutant A) (lanes 20 to 23), second (mutant B) (lanes 24 to 27), or third (mutant C) (lanes 28 to 31) ARE were incubated with or without cytosolic extracts. (D and E) Plk3 mutant probes AB (lanes 32 to 38), AC (lanes 39 to 45), and BC (lanes 46 to 52) were incubated without or with the same cytosolic extracts as in B. (E) Longer exposure of the same experiment above (D) in order to visualize the supershifted TTP/RNA complex.