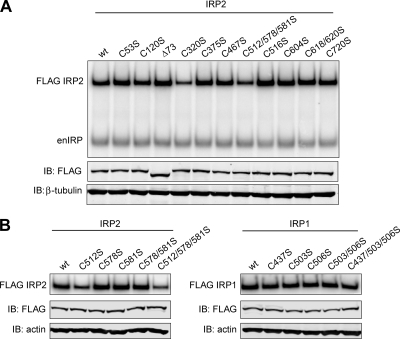
FIG. 2.
Relative RNA-binding activities of IRP1 and IRP2 cysteine mutants. (A and B) Flp-In TREx HEK293 cells stably expressing FLAG-tagged proteins were treated with tetracycline overnight to induce protein expression. RNA-binding activity was measured by EMSA by incubating cell lysates (10 μg) with a 32P-labeled ferritin-L IRE probe. Lysates from cells expressing IRP2 and IRP1 recombinant proteins were treated with 0.5% and 1% β-ME, respectively. (A) Cell lysates (12 μg) were immunoblotted (IB) and then probed simultaneously with FLAG and β-tubulin or actin antibodies. FLAG antibody was used to supershift recombinant IRP2-IRE complexes (FLAG IRP2) away from endogenous IRP-IRE complexes (enIRP). (B) Only FLAG-supershifted IRP-IRE complexes are shown (FLAG IRP2; FLAG IRP1).