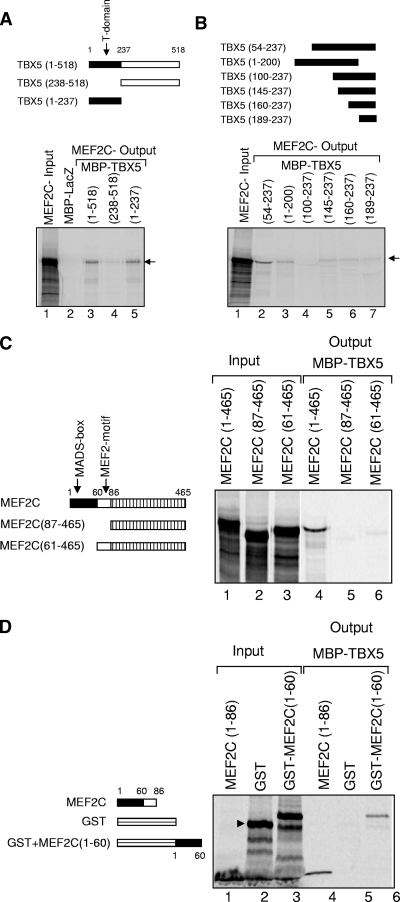
FIG. 4.
Mapping the interacting domains of TBX5 and MEF2C. (A) Fragments of TBX5 fused to MBP are shown, with the T domain (residues 1 to 237) in black, alongside an autoradiograph from a pulldown assay. Lane 1, MEF2C input; lanes 2 to 4, MEF2C output with different MBP-tagged TBX5 fragments. (B) Further mapping of residues 1 to 237 of TBX5 by using additional TBX5 deletion constructs fused to MBP. The region of TBX5 present in each case is shown in black. Lane 1, MEF2C input; lanes 2 to 7, MEF2C output following pulldown with the various MBP-tagged fragments of TBX5. (C) Full-length and deletion fragments of MEF2C, tested for binding to full-length TBX5-MBP, are shown schematically alongside an autoradiograph of the pulldown assay. Lanes 1 to 3, MEF2C input pro teins; lanes 4 to 6, outputs from these proteins following pulldown with MBP-TBX5. (D) Residues 1 to 60 of MEF2C are essential for binding to TBX5. MEF2C (residues 1 to 86) and the GST-linked MEF2C fragment (residues 1 to 60) are shown schematically, with the MADS box in black, alongside an autoradiograph of the pulldown assay. Lanes 1 to 3, input proteins; lanes 4 to 6, outputs following pulldown with MBP-TBX5.