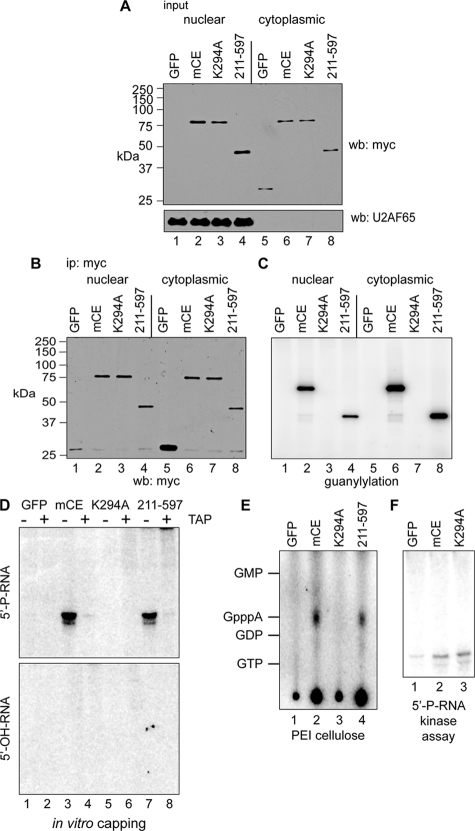
FIG. 4.
In vitro capping of 5′-monophosphate RNA by immunoprecipitated cytoplasmic capping enzyme. (A) COS-1 cells were transfected with pcDNA3 expressing myc-tagged GFP (lanes 1 and 5), full-length mCE (lanes 2 and 6), mCE with the active-site K294A mutation (lanes 3 and 7), or the guanylylation domain (211-597) alone (lanes 4 and 8). Nuclear and cytoplasmic extracts were assayed by Western blotting with antibodies to the myc tag (top) or to U2AF65 (bottom). (B) Nuclear and cytoplasmic extracts from panel A were immunoprecipitated with immobilized anti-myc monoclonal antibody, and a portion was analyzed by Western blotting with an antibody to the myc tag. (C) Anti-myc antibody-containing beads with the remaining immunoprecipitated protein from panel B were incubated with [α-32P]GTP. A portion was removed after being washed to remove unincorporated GTP and separated on an SDS-PAGE gel, and the guanylylated proteins were visualized by a PhosphorImager. (D) The remaining beads from panel C were incubated with ATP and a 23-nt RNA with a 5′-monophosphate end (5′-P-RNA; top) or 5′-hydroxyl (5′-OH-RNA; bottom). The recovered RNA was treated with (+) or without (−) TAP and visualized by PhosphorImager analysis after electrophoresis on a 15% polyacrylamide/urea gel. (E) RNAs recovered from the reaction with 5′-P-RNA in panel D were digested with P1 nuclease and separated by PEI-cellulose TLC. The mobility of unlabeled nucleotide standards is indicated on the right side of the autoradiograph. (F) Anti-myc antibody-containing beads containing immunoprecipitated GFP, mCE, or K294A from panel B were incubated with 5′-P-RNA and [γ-32P]ATP. The recovered RNA was then electrophoresed on a 15% polyacrylamide/urea gel and visualized by a PhosphorImager.