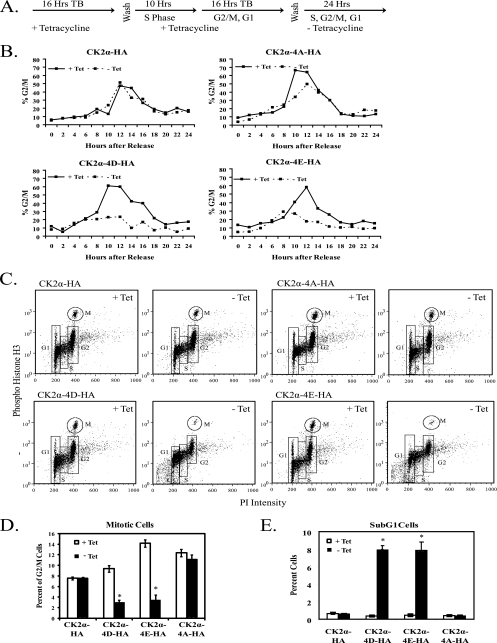
FIG. 5.
Expression of phosphomimetic mutant CK2α proteins leads to loss of mitotic cells. (A) CK2α-HA, CK2α-4D-HA, CK2α-4E-HA, and CK2α-4A-HA cells were synchronized with a double-thymidine block (TB) and then cultured in the presence (+ Tet) or absence (− Tet) of tetracycline for 24 h. (B) Cell cycle profiles of synchronized CK2α cell lines. The 0-h time point was designated the time immediately after the double-thymidine block. Cells were harvested and fixed at 2-h intervals, stained with PI, and analyzed by flow cytometry. The percentage of cells in G2/M was taken from the percentage of cells with 4N DNA content. (C) Phospho-histone H3 (serine 10) staining of cells 12 h after release from a double-thymidine block and induction of phosphorylation site mutant CK2α proteins. Cells were fixed and stained with phospho-histone H3 (serine 10) antibody, an FITC-GAR secondary antibody, and PI. Samples were analyzed by flow cytometry. (D) Graphical representation of the percentage of G2/M cells in mitosis 12 h after release from a double-thymidine block. (E) Graphical representation of the amount of cells with sub-G1 levels of DNA 12 h after release from a double-thymidine block. In panels D and E, significant differences between cells of each line in the presence or absence of tetracycline are denoted by asterisks (P > 0.05 by pairwise analysis of variance).