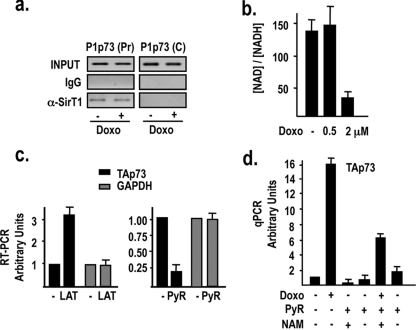
FIG. 6.
DNA damage modulates chromatin-bound hSirT1 deacetylase activity on the P1p73 promoter. (a) hSirT1 occupancy of the P1p73 promoter is unaffected by DNA damage. Chromatin from untreated and doxorubicin (2 μM)-treated U2Os cells was immunoprecipitated with either relevant control IgGs or anti-hSirT1 (α-SirT1) antibody and analyzed by P1p73 (Pr) and C PCR primers. (b) DNA damage modulates hSirT1 enzymatic activity by reducing [NAD+] levels. Free [NAD+] was calculated by measurements of [lactate] and [pyruvate] (PyR) (data not shown) in extracts from asynchronously growing U2Os cells and from U2Os cells exposed to a low nonapoptotic (0.5 μM) or apoptotic (2 μM) dosage of doxorubicin (Doxo) for 18 h. (c) Modulation of the [NAD+]/[NADH] ratio affects TAp73 expression. Cells were cultured either in the absence or in the presence of l-lactate (10 mM) or pyruvate (30 mM) for 24 h, and both TAp73 and GAPDH (glyceraldehyde-3-phosphate dehydrogenase) mRNA levels were analyzed by RT-PCR as described in the legend of Fig. 1b. (d) Reactivation of hSirT1 deacetylase activity by pyruvate treatment abolishes the DNA damage-induced activation of the endogenous P1p73 promoter. Untreated and doxorubicin (2 μM)-treated cells were cultured either in the absence or in the presence of pyruvate (30 mM) and the combination of pyruvate and NAM (10 mM) for 24 h, and TAp73 mRNA levels were analyzed by real-time quantitative PCR (qPCR).