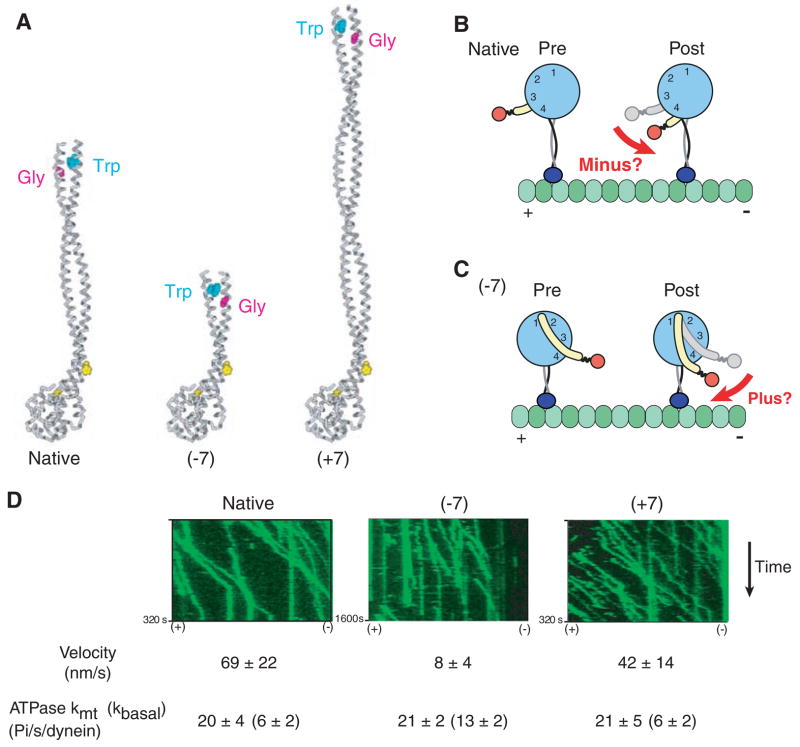
Fig. 3.
Rotation of the AAA+ ring by insertion or deletion of stalk sequence does not affect the direction of dynein movement. (A) Model of native dynein stalk (at left), showing the pair of conserved prolines (yellow spacefill) marking the MTBD and the conserved Trp-Gly pair that mark the top of the stalk coiled coil. Removal of seven heptads from the middle of the dynein stalk (−7) or insertion of seven heptads from the dynein stalk of Drosophila cytoplasmic dynein (+7) rotates the position of the Trp-Gly pair by 180° with respect to the microtubule-binding domain. (B) Cartoon of a model for how conformational changes in the AAA+ ring lead to minus end–directed motion [adapted from (8)] via rotation of the linker domain (yellow) toward the minus end. (C) Cartoon showing how the model in (B) predicts that rotation of the AAA+ ring by 180° due to a change in the length of the stalk should produce a plus end–directed motor. (D) Single-molecule fluorescence assay for the directionality of dimeric S. cerevisiae dynein constructs [based on GST-Dyn1331kD (18)] with different lengths of stalk. Kymographs of tetra-methyl rhodamine–labeled dynein constructs from a single axoneme in the assay (green) show that all constructs move unidirectionally toward the minus end of the microtubule. The orientation of the axoneme was determined using fluorescent Cy5-labeled kinesin (see movies S2 to S4). Single-molecule velocities (means ± SD) and ATPase measurements (means ± SD) were determined as described in the supporting online material.