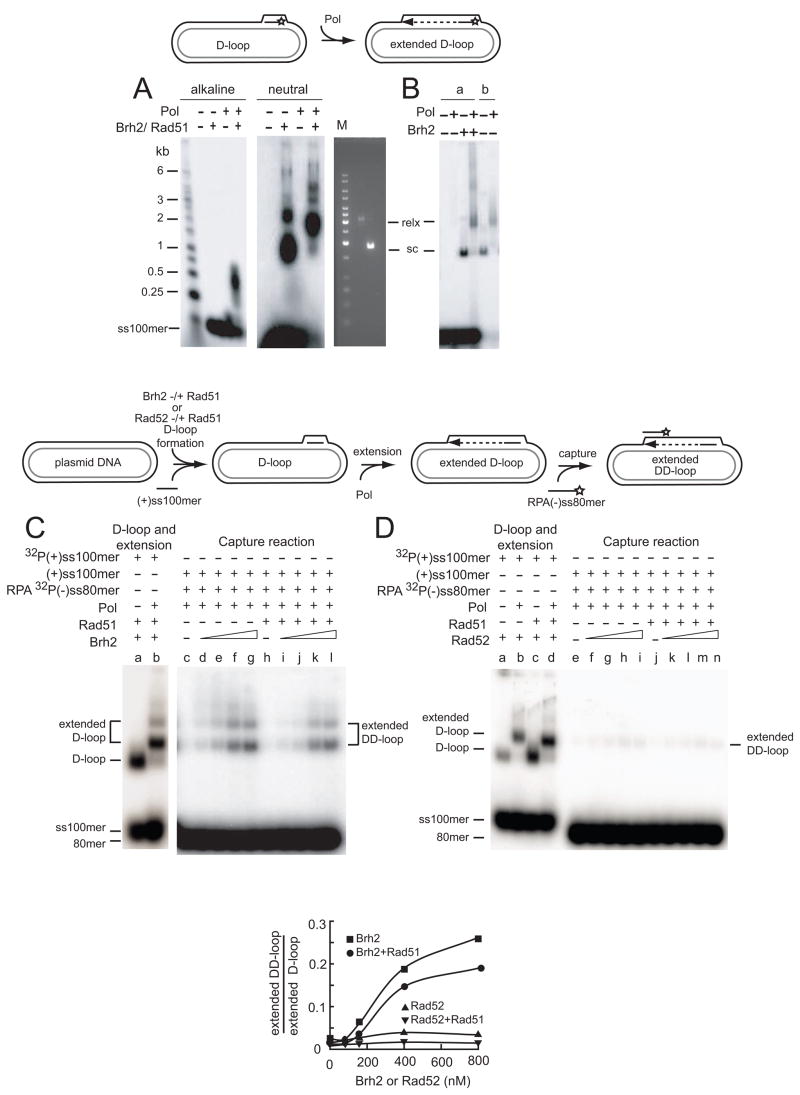
Figure 5.
Brh2-promoted duplexed D-loop formation coupled with DNA synthesis. The schematic depicts use of the third (invading) strand in a D-loop as a primer for DNA synthesis. A. D-loops were formed in standard reactions optimized for yield in which 6 nM 32P-labeled (with star) ss100mer (+ strand) had been preincubated with 750 nM Rad51 in the presence of 2 mM ATP, 2 mM CaCl2 and 18 nM pBluescript plasmid DNA had been pre-mixed with 400 nM Brh2. After D-loop formation, deoxynucleoside triphosphates and DNA polymerase (Pol) were added. Following incubation for 30 min the mixtures were split, deproteinized and electrophoresed under neutral (left panel) or denaturing conditions (30 mM NaOH, middle panel). A gel run in parallel with plasmid DNA in supercoiled (sc) or relaxed form (relx) using wheat germ topoisomerase (Promega) was stained with ethidium bromide. B. In the lanes indicated under a, D-loop reactions were performed with Brh2 as above, but without Rad51, followed by addition of DNA polymerase. In the lanes indicated under b, preformed purified D-loop DNA free of unincorporated 32P-labeled ss100mer was used in reaction with DNA polymerase. C. The schematic depicts the stepwise protocol for D-loop formation and DNA-synthesis-coupled second-end capture. D-loop formation controls were performed with Brh2 (400 nM) and Rad51 (750 nM) with or without Pol (lanes a, b) as in A using 32P-labeled ss100mer (+ strand) to gauge the level of D-loop formation. In the second end capture determinations with increasing levels of Brh2 in the presence or absence of 750 nM Rad51 (lanes c–l) D-loop formation was initiated in the same way, but with the use of unlabeled ss100mer (+ strand). After the extension period with DNA polymerase, 32P-ss80mer (− strand) coated with RPA was added and mixtures incubated an additional 30 min. Brh2 was added as follows: lanes c, h-no Brh2; lanes d, i-80 nM; lanes e, j-160 nM; lanes f, k-400 nM; lanes g, l-800 nM. Product formation was quantified by comparison of the extended duplexed D-loop product against the standard of extended D-loops (lane b) and expressed graphically below as extended DD-loop/extended D-loop. D. Reactions were performed as in C except that Rad52 was used instead of Brh2. In the controls (lanes a–d) D-loop formation by Rad52 (200 nM) and Rad51 (750 nM) and extension by Pol were gauged using 32P-labeled ss100mer (+ strand). Second-end capture was performed as above with increasing Rad52 as follows: lanes e, j-no Rad52; lanes f, k-80 nM; lanes g, l-160 nM; lanes h, m-400 nM; lanes I, n-800 nM. Product formation was quantified as in C by comparison of the extended duplexed D-loop product against the standard of extended D-loops minus or plus Rad51 (lane b or d, respectively) and expressed graphically below.