Abstract
Microemulsions (ME)—nanostructured systems composed of water, oil, and surfactants—have frequently been used in attempts to increase cutaneous drug delivery. The primary objective addressed in this work has been the development of temperature-sensitive microemulsion gel (called gel-like ME), as an effective and safe delivery system suitable for simultaneous topical application of a hydrophilic vitamin C and a lipophilic vitamin E. By changing water content of liquid o/w ME (o/w ME), a gel-like ME with temperature-sensitive rheological properties was formed. The temperature-driven changes in its microstructure were confirmed by rotational rheometry, viscosity measurements, and droplet size determination. The release studies have shown that the vitamins’ release at skin temperature from gel-like ME were comparable to those from o/w ME and were much faster and more complete than from o/w ME conventionally thickened with polymer (o/w ME carbomer). According to effectiveness in skin delivery of both vitamins, o/w ME was found the most appropriate, followed by gel-like ME and by o/w ME carbomer, indicating that no simple correlation between vitamins release and skin absorption could be found. The cytotoxicity studies revealed good cell viability after exposure to ME and confirmed all tested microemulsions as nonirritant.
Key words: antioxidant, microemulsion gel, rheology, skin permeation, vitamin
INTRODUCTION
In attempts to increase cutaneous drug delivery, microemulsions (ME) have been more and more frequently employed over recent years. ME are nanosized mixtures of water, oil, and surfactants that are transparent, single phase, optically isotropic, and thermodynamically stable. They have been shown to be superior to conventional vehicles like emulsions or hydrogels for dermal delivery of hydrophilic and especially lipophilic drugs. The favorable drug delivery properties of ME are attributed mainly to their excellent solubilizing properties. They can also act as penetration enhancers, depending on the nature of the oil and surfactant constituents (1).
Besides optimizing the formulation to maximize cutaneous drug bioavailability, it is also important to ensure that it is aesthetically acceptable to patients, is easy to use, and adheres to skin sufficiently (2). Optimizing rheological behavior is therefore one of the crucial steps in development of dermal drug delivery systems. Depending on their composition, ME exhibit a number of structures, varying from dispersed droplets of different shape and size to liquid lamellar crystals (3). It is generally known that from o/w ME under certain conditions upon the addition of specific amounts of water transparent isotropic gels can be formed (4). Unfortunately, these water-induced gel structures are frequently easily disturbed and the addition of drug molecules can considerably affect their stability. The more usual way to optimize the rheological behavior of topical ME is addition of thickening agent that increases the viscosity of the system without affecting its stability and spontaneous formation (5). However, finding an appropriate thickener is a time-consuming task since the selection is done empirically and numerous thickeners have to be screened. Thickened ME are usually composed of two distinct structural elements, a network formed by thickener in the outer phase that coexists with the microemulsion droplets (4).
Even though sunlight is indispensable for life, it is well known that UV regions of the spectrum are linked to skin disorders ranging from mild inflammatory effects to serious diseases like skin cancer (6). Cells are equipped with a variety of mechanisms that constantly monitor and repair UV-induced damage, the most important being nucleotide excision repair systems and enzymatic and nonenzymatic antioxidants. Nevertheless, excessive exposure to sunlight can still cause depletion of skin repair mechanisms and the development of effective strategies to support the cells’ protection mechanisms is important in achieving protection against and therapy of cutaneous disorders (7).
Because the most important nonenzymatic aqueous- and lipid-phase antioxidants—vitamins C (l-ascorbic acid) and E (α-tocopherol), respectively—can only be provided exogenously, it is beneficial to enhance oral supplementation by topical application for extra protection of the skin (8). In cells, vitamins C and E act synergistically to provide antioxidant protection. It has been shown that a topical combination of l-ascorbic acid with α-tocopherol gives fourfold protection against UV-induced erythema, compared to twofold protection by either vitamin alone (8–11).
The major challenge for topical delivery of antioxidants is development of formulations that could stabilize them and that provide a sufficient increase in their penetration into the skin. Furthermore, delivery systems that allow simultaneous incorporation of antioxidants with different lipophilicities such as ME or liposomes are desirable (12–14).
The primary objective addressed in this work has been the development of temperature-sensitive ME gel (called gel-like ME), composed of uniquely pharmaceutically acceptable components, as an effective and safe delivery system suitable for simultaneous topical application of a hydrophilic and a lipophilic vitamin. We formulated temperature-sensitive ME gel with a suitable consistency for topical application at 20°C that changes into liquid ME at skin temperature and thus accelerates vitamins release. First, we studied temperature-driven changes of gel-like ME in comparison to liquid o/w ME (called o/w ME) and conventionally thickened o/w ME (called o/w ME carbomer) using rheological and droplet size measurements. Second, the effectiveness of all three formulations as a vehicle for simultaneous topical delivery of vitamins C and E was investigated using in vitro skin permeation test. Finally, the in vitro toxicity of model cell cultures exposed to ME was evaluated by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay and by fluorescence microscopy.
MATERIALS AND METHODS
Preparation of ME
Isopropyl myristate was obtained from Fluka Chemie, Switzerland and used as the lipophilic phase. Tween 40—polyoxyethylene (20) sorbitan monopalmitate (Fluka Chemie, Switzerland)—was used as surfactant and Imwitor 308—glyceryl caprylate (Condea, Germany)—as cosurfactant. Purified water was used as the hydrophilic phase. α-Tocopherol (vitamin E, viscous liquid, molecular weight 430.72 g/mol) and ascorbic acid (vitamin C, white crystalline powder, molecular weight 176.12 g/mol) were from Fluka, Switzerland. The composition of tested ME is given in Table I.
Table I.
Composition of gel-like ME, o/w ME, and o/w ME carbomer
| Component | % w/w | ||
|---|---|---|---|
| gel-like ME | o/w ME | o/w ME carbomer | |
| Tween 40 | 14.79 | 14.79 | 14.42 |
| Imwitor 308 | 14.79 | 14.79 | 14.42 |
| Isopropyl myristate | 9.86 | 24.65 | 24.03 |
| Purified water | 59.16 | 44.37 | 43.26 |
| Vitamin E | 1.00 | 1.00 | 1.00 |
| Vitamin C | 0.40 | 0.40 | 0.40 |
| Carbomer | / | / | 2.47 |
All ME were prepared in the same way. The surfactant and cosurfactant were blended in a 1:1 mass ratio to give the surfactant mixture. Isopropyl myristate and distilled water were then added and mixed with a magnetic stirrer for 5 min at room temperature. Vitamins C and E were incorporated by stirring with a magnetic stirrer for 30 min. o/w ME carbomer was prepared by adding carbomer (Carbopol 974 PNF, BF Goodrich, Belgium) to o/w ME containing vitamins and stirred with a magnetic stirrer for 30 min. The ME were left covered for at least 24 h before use. The final vitamin content was always in the range 95–105% of the amount added to ME.
Rheological Measurements
The rheological characteristics were determined with a Rheolab MC 100 Paar Physica, controlled shear rate rheometer at 20°C and 32°C. The geometry used was a stainless steel cone/plate system MK22 (r = 25 mm, θ = 1°). All measurements were made in triplicate.
The absolute dynamic viscosity of ME was determined in triplicate in a temperature range 20–32°C using a SV-10 Vibro Viscosimeter, A&D Company, Japan. The temperature coefficient of viscosity was calculated:
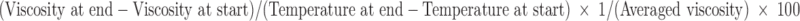 |
1 |
and expressed in [%/°C].
Size Determination
Dynamic light scattering measurements of the hydrodynamic radius and polydispersity of the ME structures were performed at 20°C and 32°C using a Nano ZS, Malvern Instruments. ME samples were thermostated 5 min before measurement. The viscosity of the samples was determined as described in section “Rheological measurements” and the refractive indexes were measured using refractometer Carl Zeiss.
Solubility of Vitamins C and E
Excess vitamin C (0.5 g) or vitamin E (3 g) was added to 4 g of ME or their components. The systems were allowed to reach equilibrium by stirring at 25 ± 1°C for 24 h. Samples were then centrifuged at 28,000 rpm for 20 min at 5°C to separate excess vitamin from the formulations. Under test conditions, vitamin E dissolved completely in surfactant mixture and isopropyl myristate. Vitamin E was therefore mixed with surfactant mixture to give mass fractions ranging from 10% to 90% in 10% steps and left to equilibrate as described above. The same procedure was repeated for vitamin E solubility in isopropyl myristate. After dilution and filtration, samples were analyzed by high performance liquid chromatography (HPLC). All samples were analyzed in triplicate.
Release Studies
Vitamins C and E release rates from formulations were measured through cellulose acetate membrane (pore size 0.45 μm, Sartorious, Goettingen, Germany) soaked in receptor solution 24 h before experiments. Franz diffusion cells with a diffusion area of 0.785 cm2 and 8 ml of receptor volume were used. To take account of the very low solubility of vitamin E and to ensure its stability, isopropyl myristate solution with 0.5% (w/w) Tween 40 and 0.5% (w/w) Imwitor 308 was demonstrated to be a suitable receptor phase. For vitamin C, 0.9% NaCl was used as receptor fluid. Five hundred milligrams of ME was dosed in the donor compartment. The system was kept in a temperature-controlled water bath to maintain the temperature in the donor compartment at 32°C or 20°C and the receptor phase was continuously stirred. At predetermined time intervals, 0.3 ml samples were taken and replaced by the same volume of fresh preheated receptor. Vitamin concentrations were determined by HPLC. Each experiment was done in quadruplicate.
Cumulative amount of vitamin released was plotted against square root of time:
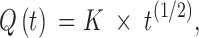 |
2 |
where Q(t) is the cumulative amount (nmol/cm2) of vitamin released in time t (<60%), K (nmol/(h(1/2) cm2)) is the kinetic constant indicative of vitamin release rate, and t(1/2) is square root of time. The degree of linearity Q(t) = f (t(1/2)) was checked for all formulations for both vitamins and Pearson’s coefficient was always above 0.993.
Permeation Studies
Pigs’ ears were obtained from the local slaughterhouse. The skin was kept frozen until use. Before use, it was briefly washed under tap water, hairs were removed, and the skin was sliced (thickness <1 mm). Skin slices were mounted on Franz diffusion cells and filled with receptor fluid the evening before the experiment and left to equilibrate overnight in water bath maintaining skin surface temperature at 32°C. Before experiment, the whole receptor compartment was emptied out and refilled with fresh preheated medium. Eight milliliters of 0.9% NaCl with 3% of chicken egg albumin (Sigma Aldrich, Germany) was used as the receptor fluid. The area available for diffusion was 0.785 cm2. One gram of formulation was spread on the skin surface. At predetermined time intervals (30, 60, 120, 180, 240, and 360 min), 1 ml of sample was taken from the receptor compartment and replaced by fresh medium. Vitamins were extracted from collected samples with MeOH and analyzed by HPLC. After 6 h, the formulation was removed and the skin surface cleaned. Epidermis was separated from dermis by heating the samples with hair dryer for 15 s and separating analyzed skin layers with the help of two pairs of tweezers. After separation, the epidermis and dermis were cut into small pieces and vitamins extracted with MeOH. Samples were analyzed by HPLC. The experiments were conducted in a temperature-controlled water bath, resulting in a membrane surface temperature of 32°C in quadruplicate.
The skin permeation rate at steady-state (steady-state flux J; nmol/cm2 h) was calculated from the slope of the linear portion of the plots of cumulative vitamin C permeation per unit of skin surface area against time.
HPLC Analysis
HPLC analysis was carried out with an Agilent 1200 series HPLC system. Chromatographic conditions for vitamin E: a 120 × 4-mm ID column packed with 5 μm Nucleosil C18 as stationary phase; the mobile phase was methanol:acetonitrile 70:30. The flow rate was 1.5 ml/min. UV detection was at 291 nm. The limit of quantification (LOQ) for chromatographic determination of vitamin E was determined from the calibration curve and was 1.25 μM; the limit of detection (LOD) was 0.412 μM.
Chromatographic conditions for vitamin C: a 250 × 4-mm ID column packed with 5 μm Nucleosil C18-NH2 as stationary phase; the mobile phase was methanol:acetonitrile:0.02 M phosphate buffer pH 3.5 (20:30:50). The flow rate was 1 ml/min. UV detection was at 243 nm. LOQ for chromatographic determination of vitamin C was determined from the calibration curve to be 22.7 μM; LOD was 7.5 μM.
Cell Cytotoxicity
Human embryonic kidney cells (HEK293, ATCC) were cultured in Dulbecco’s modified essential medium (Sigma, Germany) supplemented with 10% heat inactivated fetal calf serum (Gibco, Invitrogen, Carlsbad, CA, USA), 2 mM l-glutamine (Sigma, Germany), and 100 U/ml penicillin/streptomycin (Sigma, Germany). All cells were incubated in humidified atmosphere at 37°C in 5% CO2.
MTT assay was performed according to the method of Mosmann with modifications for HEK293 cells proposed by Kristl et al. (15). Yellow 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide, a tetrazole, is metabolically reduced to purple formazan. MTT is cleaved to formazan by succinate–tetrazolium reductase system which belongs to the mitochondrial respiratory chain and is active only in viable cells. Since the amount of formed formazan is directly proportional to the number of living cells in culture, the intensity of produced color is an indication of the viability of the cells. Cells were seeded in 96-well plates (2.5 × 104 cells/well, 100 μl of cell culture medium) and when 40% confluence was reached, 10 μl of test dispersion were added for 24 h. Test dispersions were prepared by diluting ME in cell culture medium (1:200) to achieve the final concentration of ME 450 μg/ml. Only formulations without vitamins were tested, since vitamins C and E are both strong antioxidants, capable of reducing MTT to formazan. In negative control experiments, cells were treated with cell culture medium. SDS solution diluted in cell culture medium to give final concentration of 450 μg/ml was used as a positive control. After 21 h, 11 μl of the MTT dissolved in the assay medium (2 mg/ml) were put in each well and cells were incubated at 37°C and 5% CO2 during 3 h. The insoluble purple formazan product was extracted from the cells by acidic isopropanol into a colored solution. The absorbance of this colored solution was quantified by measuring at 570 nm in an automated plate reader (Safire2™ Tecan, Switzerland). Average cell viability of treated cells was expressed as percentage of absorbance of ME-treated cells as follows:
Cell viability = ((AS − AS0)/(AC − AC0)), where AS is the absorbance of treated cells, AC is the absorbance of untreated cells (control), AS0 is the absorbance of ME diluted in medium without cells, and AC0 the absorbance of the medium alone. All tests were done in triplicate.
Cell growth and morphology were observed using an inverted phase-contrast microscope (Olympus CKX41, Japan). Cells were plated on square glass cover slips and incubated in 6-well plates overnight. Following the incubation with different ME (final concentration 450 μg/ml of cell culture medium), the cells were fixed with ice-cold 4% paraformaldehyde in phosphate buffered saline pH 7.4 for 10 min and permeabilized for 10 min in 0.1% Triton X-100 (both Sigma, Germany). Cell nuclei were visualized by staining with a DNA intercalating dye Hoechst 33342 (Riedel de Haen, Germany, 5 μg/ml) for 30 min in the dark. Actin fibers were stained with Phalloidin TRITC (Sigma, USA, 1:40). After staining, the cover slips were removed from the wells, mounted on a slide, and viewed using 360/420 nm (Hoechst) and 535/635 nm (Phalloidin—TRITC) excitation/emission filter sets.
Data Analysis
Influence of formulation on vitamins’ solubility, release rate, skin permeation, and on cell viability was evaluated by one-way analysis of variance with KaleidaGraph® software package. Bonferroni’s test was used for post hoc comparisons. Significance was tested at the 0.05 level of probability.
RESULTS AND DISCUSSION
Viscosity and Rheological Behavior
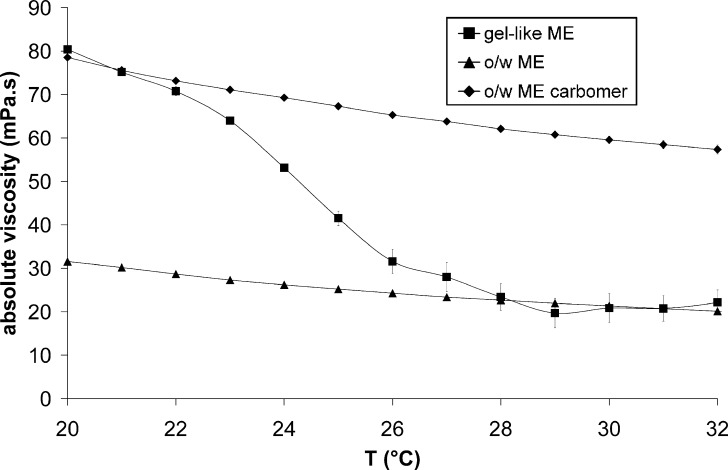
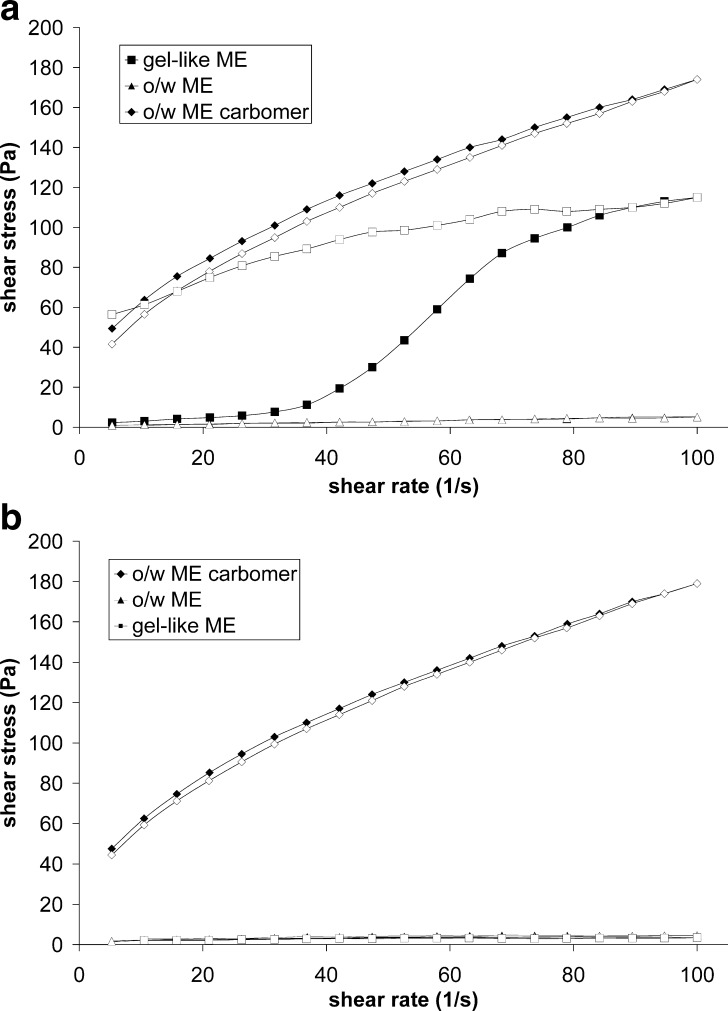
Rheological analysis is one of the most frequently used techniques for characterization of ME structure at macroscopic level. Rheological measurements of tested formulations demonstrated that their flow behavior depends on composition and on surrounding temperature (Figs. 1 and 2a, b).
Fig. 1.
Temperature dependence of absolute viscosity for gel-like ME, o/w ME, and o/w ME carbomer
Fig. 2.
Flow curves of gel-like ME, o/w ME, and o/w ME carbomer: a at 20°C and b at 32°C. Full symbols represent ascending part of a curve
At 20°C, gel-like ME exhibits almost threefold higher viscosity than o/w ME that was used as a comparison (Fig. 1). Although the topical application of o/w ME is possible, a slightly increased viscosity of the vehicle, like in the case of gel-like ME, is desirable. For comparison, we also formulated o/w ME carbomer with approximately the same viscosity at 20°C as that of gel-like ME. This was done conventionally by adding a suitable thickener to o/w ME. The choice of appropriate thickener was done empirically by screening different polymers such as xanthan, hydroxypropyl methyl cellulose, microcrystalline cellulose, locust bean gum, carbomer, and sodium alginate. Only with carbomer, a synthetic high molecular weight acrylic acid polymer, a stable system with appropriate viscosity could be formed that was called o/w ME carbomer.
As can be seen from Fig. 1, the viscosity of all three systems in the temperature range 20–32°C is decreasing. In the case of o/w ME and o/w ME carbomer, the decrease is linear with time. Moreover, their temperature coefficients of viscosity calculated using Eq. 1 were approximately the same (−3.5 ± 0.1%/°C and −2.8 ± 0.2%/°C for o/w ME and o/w ME carbomer, respectively). They are close to the temperature coefficients of other liquids and semisolids (e.g. olive oil, silicone oil, water, honey). All these data proved that a small decrease in viscosity is a typical reaction of many systems when exposed to slightly increased temperature. On the contrary, the viscosity of gel-like ME decreases drastically with temperature (its temperature coefficient of viscosity is −11.8%/°C) and at 32°C, its viscosity is the same as that of o/w ME. The rapid changes in viscosity, reflected in high temperature coefficient of viscosity, are usually associated with the change in microstructure of ME.
The changes in viscosity following ME exposure to shear stress shown on Fig. 2a, b (at 20°C and 32°C, respectively) additionally confirmed the temperature driven changes in rheological behavior of gel-like ME that were on contrary not observed either in o/w ME or in o/w ME carbomer.
At 20°C, gel-like ME expressed rheopectic behavior, rarely seen in pharmaceutical systems (Fig. 2a). It has been proven by small angle X-ray scattering measurements that at rest, gel-like ME consists of lamellar structures (16) that are the consequence of increased interactions between highly hydrated surfactant chains (17). At higher shear rates, the aqueous layer of surfactants is disrupted, which is reflected in increased resistance to flow (Fig. 2a). However, at a skin temperature, the rheological behavior of gel-like ME changed to that of o/w ME that are characterized as low-viscosity Newtonian fluids, indicating the destruction of the gel-like structure (Fig. 2b). As mentioned above, o/w ME that differs from gel-like ME only in water content is a stable, ideal Newtonian fluid at 32°C as well as at 20°C. The addition of carbomer to o/w ME changed its rheological behavior from Newtonian to thixotropic (Fig. 2a). The shear stress applied caused the macromolecules of carbomer to align in the direction of the shear stress vector, which resulted, in opposition to gel-like ME, in reduced resistance to flow. Increase in temperature to 32°C did not affect its rheological behavior (Fig. 2b). The addition of vitamins did not influence the viscosity of gel-like ME (75.6 mPa.s at 20°C)
Droplets Size
Table II shows the hydrodynamic radius of the droplets of ME samples obtained from photon correlation spectroscopy (PCS) measurements at 20°C and 32°C. Polydispersity index is listed only for samples that had a single peak in the intensity chart.
Table II.
Droplet Size and Polydispersity Index (PI) for gel-like ME, o/w ME and o/w ME carbomer at 20°C and 32°C
| Formulation | T = 20°C | T = 32°C | ||
|---|---|---|---|---|
| Size (nm) | PI | Size (nm) | PI | |
| gel-like ME | / | / | 1.88 ± 0.11 | 0.279 |
| o/w ME | 1.23 ± 0.07 | 0.242 | 2.36 ± 0.06 | 0.213 |
| o/w ME carbomer | 0.877 ± 0.041 | / | 1.32 ± 0.04 | / |
PI polydispersity index
No structures in the size range 0.1–10,000 nm were observed in gel-like ME at 20°C, although the scattering of the sample was good. As described previously, gel-like ME is composed of double-layer lamellar phases (16) that could not be measured by PCS. However, it is very interesting to note that at 32°C small droplets were detected, confirming our hypothesis that increase in temperature lead to structural reorganization of gel-like ME into o/w ME consisting of fine oil droplets dispersed in aqueous phase.
As already confirmed by Podlogar et al. (17), o/w ME also has very small size aggregates, which makes this system optically transparent. Increase in temperature to 32°C does not considerably affect droplets size (Table II). In the case of o/w ME carbomer, the additional peak due to the presence of polymer aggregates (451.3 ± 105 at 20°C and 872.7 ± 132 at 32°C) was noticed. Nevertheless, the droplets size was comparable to that of o/w ME.
Solubility Studies
The solubility of vitamins C and E was determined in tested ME and in particular components (Table III). Significant differences (p < 0.05) in solubility of both vitamins in gel-like ME, o/w ME and o/w ME carbomer were seen that were mostly induced by different composition of the vehicles. Vitamin C is a freely water soluble drug. Its solubility in other components of o/w ME is very limited (Table III), leading to conclusion that it must be located mainly in the aqueous phase of ME. Accordingly, its solubility in ME is decreased with lower water content (gel-like ME vs. o/w ME; Table III). On the other hand, vitamin E is a poorly water soluble drug but completely miscible with the oily and surfactant phases. Consequently, in contrast to vitamin C, its solubility in ME decreases with increased water content. Addition of carbomer slightly lowered vitamin E solubility, probably due to its creation of a more hydrophilic environment (18). This would also account for the small increase of vitamin C solubility in o/w ME carbomer.
Table III.
Solubility of Vitamins C and E at 25 ± 1°C
| Formulation | Solubility (mg/g) ± SD | |
|---|---|---|
| Vitamin C | Vitamin E | |
| o/w ME | 85.5 ± 2.6 | 134 ± 5 |
| o/w ME carbomer | 96.9 ± 1.7* | 107 ± 3* |
| gel-like ME | 170 ± 4* | 74.6 ± 0.1* |
| Water | 335 ± 2* | 20.9* |
| Isopropyl myristate | 0.0113 ± 0.0009* | Completely miscible |
| Emulsifiers | 10.9 ± 0.7* | Completely miscible |
*p < 0.05 compared to o/w ME
Release Studies
Several parameters can influence the mechanism of drug release from ME, such as type of ME, its internal structure, viscosity, vitamin solubility, and vitamin hydrophilicity/lipophilicity. In our study, all formulations were of the same type (o/w), but they differed in their rheological properties and microstructure (Figs. 1 and 2; Table II). Moreover, they were simultaneously loaded with hydrophilic vitamin C and lipophilic vitamin E whose solubility in the vehicle depended on formulation.
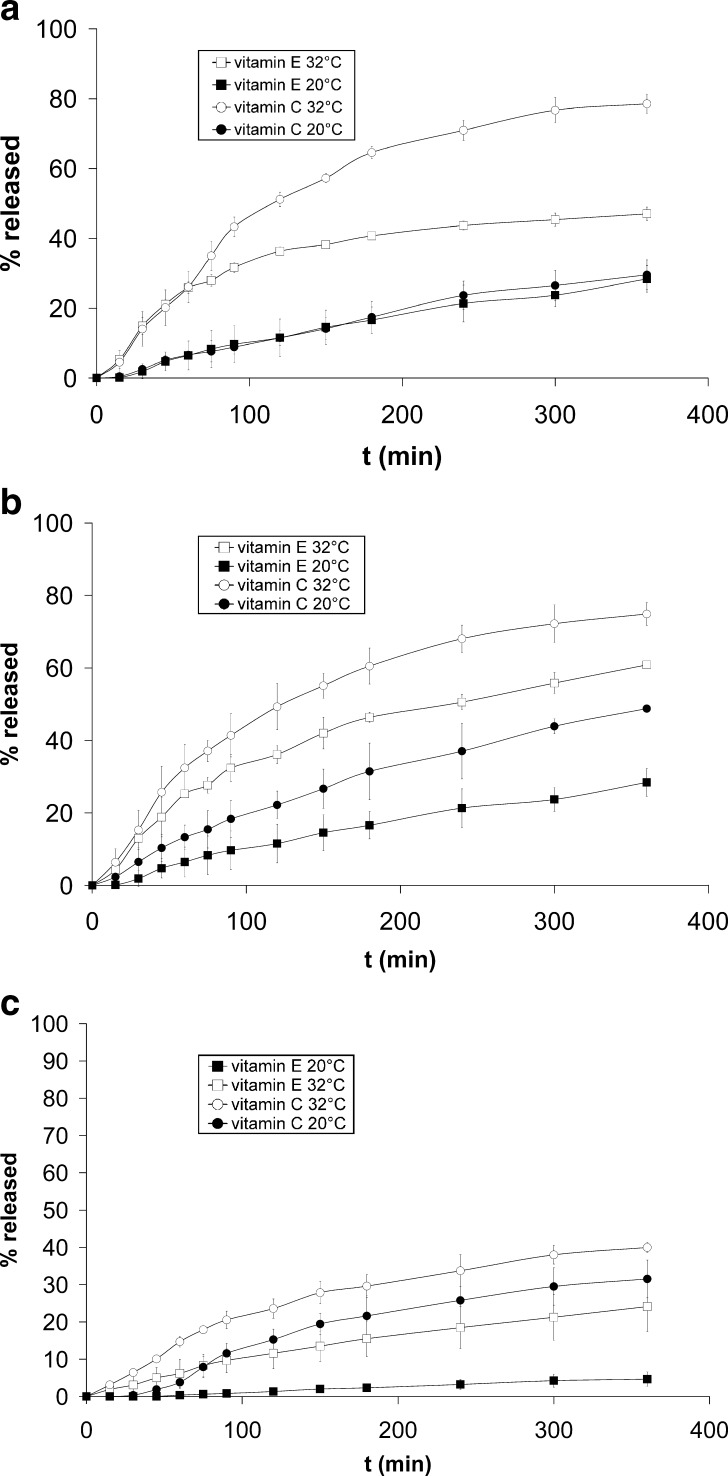
The release profiles of vitamins C and E from tested ME (Fig. 3a–c) were determined at 20°C and 32°C. The release rate constant (K; Eq. 2) was calculated from the slope of the linear portion of the plots of cumulative drug released against t1/2 and expressed in nmol/(cm2 h1/2) in order to facilitate comparison between vitamin E and C release since their molar concentration was the same in all vehicles (23 μmol/g of formulation).
Fig. 3.
Release profiles of vitamins C and E at 20°C and 32°C from: a gel-like ME, b o/w ME, and c o/w ME carbomer
From gel-like ME at 32°C, 75% of vitamin C was released in 6 h, characteristic time elapsing between two subsequent topical applications. The amount of vitamin E released (Fig. 3a) and its release rate (Table IV) was considerably lower. Vitamin E, being lipophilic, is incorporated in the inner phase of ME and has to partition from oily droplets into the continuous phase before being released to the receptor solution, whereas vitamin C is located mainly in the outer aqueous phase. At 20°C, no difference between vitamin E and C release from gel-like ME was observed (Fig. 3a; Table IV), which is consistent with our previous conclusions that gel-like ME at 20°C does not have a typical microstructure of oily droplets dispersed in aqueous medium. In contrast to gel-like ME at 20°C, the difference in vitamins C and E release from o/w ME was clearly seen (Fig. 3b; Table IV). The comparison of release profiles and release rates of vitamin C at 32°C (Fig. 3a, b; Table IV) revealed no difference between the gel-like and o/w ME, since the structure of the former one has been shown to be destroyed at physiologic temperature (Fig. 2; Table II). However, at 32°C, vitamin E was released more slowly from gel-like ME than from o/w ME. The possible explanation is that the former one has higher percentage of water, which constitutes a diffusion barrier for vitamin E.
Table IV.
Release Rate Constants (K) of Both Vitamins from Different ME Calculated from the Linear Part of Release Profiles Using the Time Square Root Model (Eq. 2) at 20°C and 32°C
| Formulation | K (nmol/(h1/2 cm2)) | |
|---|---|---|
| Vitamin C | Vitamin E | |
| o/w ME 32°C | 101 ± 10** | 59.9 ± 1.7** |
| gel-like ME 32°C | 110 ± 16** | 43.2 ± 3.0*,** |
| o/w ME carbomer 32°C | 52.9 ± 7.4* | 28.0 ± 3.8* |
| o/w ME 20°C | 44.6 ± 3.8* | 31.2 ± 3.3* |
| gel-like ME 20°C | 20.4 ± 4.8*,** | 25.8 ± 1.9* |
| o/w ME carbomer 20°C | 26.6 ± 2.2*,** | 3.95 ± 0.34*,** |
*p < 0.05 compared to o/w ME at 32°C; **p < 0.05 compared to o/w ME at 20°C
Release of both vitamins from o/w ME carbomer at 32°C was incomplete and very slow in comparison to o/w ME and gel-like ME (Fig. 3a–c; Table IV). As for other two tested ME with decrease of temperature to 20°C, the release of both vitamins from o/w ME carbomer was additionally slowed down (Fig. 3c; Table IV).
Permeation Studies
The permeation experiments were performed using pig ear skin as barrier. When considering the penetration of a vitamin from different formulations into the skin apart from vehicle–vitamin interactions (proven by solubility and release experiments), influence of the carrier on the skin barrier has to be considered. In the case of ME especially surfactants may alter the structure of the skin and modify the skin absorption of vitamins. Although in the present study all tested ME contained approximately the same amount of surface active agents (Table I), the differences among tested ME were observed (Table V).
Table V.
Vitamins’ Amounts in Epidermis and Dermis After 6 h of Contact with gel-like ME, o/w ME, and o/w ME carbomer
| Formulation | Vitamin C | Vitamin E | ||
|---|---|---|---|---|
| Epidermis (nmol) | Dermis (nmol) | Epidermis (nmol) | Dermis (nmol) | |
| gel-like ME | 14.9 ± 1.5 | 1.14 ± 0.03* | 33.3 ± 7.7 | 338 ± 9 |
| o/w ME | 15.1 ± 2.9 | 2.48 ± 0.27 | 37.4 ± 9.2 | 415 ± 22 |
| o/w ME carbomer | 15.7 ± 4.2 | 1.09 ± 0.20* | 40.1 ± 1.8 | 315 ± 65 |
*p < 0.05 compared to o/w ME
Two permeation parameters were evaluated: vitamin accumulation in the skin layers and their permeation through the skin into receptor fluid. Vitamin contents in epidermis and dermis were analyzed separately. Gel-like ME delivered in the epidermis approximately the same amount of both vitamins than other two tested ME (p = 0.98 and 0.58 for vitamins C and E, respectively), but molar concentrations of vitamin E were on average twofold higher than those of vitamin C (Table V).
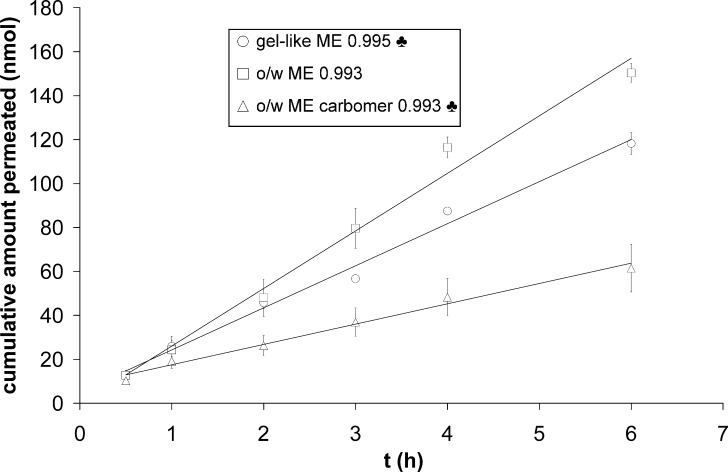
Concerning delivery into dermis and receptor fluid, gel-like ME delivered fewer vitamins than o/w ME, but more than o/w ME carbomer (Table V; Fig. 4). Significant amounts of vitamin E were found in dermis (Table V) and none in the receptor fluid. Vitamin E can bind to skin tissue where it forms a very strong reservoir (19) and although the solubility of vitamin E in receptor fluid was improved by adding albumin, its partitioning from skin to receptor fluid was still not favored. Overall, molar concentrations of vitamin C in the dermis were up to 200-fold lower than those of vitamin E (Table V). It seems that vitamin C, in contrast to vitamin E, does not favor accumulation in dermis.
Fig. 4.
Vitamin C permeation profiles from gel-like ME, o/w ME, and o/w ME carbomer. Lines: zero-order kinetics. Pearson’s coefficients are given next to the symbols in the legend. ♣ p < 0.05 compared to o/w ME
o/w ME enabled a higher delivery of vitamin E in dermis than gel-like ME, although the differences were not statistically significant. This could be, apart from lower amount of isopropyl myristate, a well-known penetration enhancer (20), also attributed to sustained release of vitamin E from gel-like ME (Fig. 3a, b). o/w ME carbomer also delivered less vitamin E in dermis than o/w ME. Again, this effect can be attributed mainly to unfavorable partitioning of vitamin E from the dispersed oily phase to the continuous aqueous phase.
In all cases, vitamin C was found in the receptor solution after 30 min (Fig. 4). Steady-state fluxes were obtained from the slope of the linear part of permeation profiles (Table VI). Less vitamin C permeated the skin from gel-like ME than from o/w ME (p < 0.001). Moreover, its permeation rate was lower from gel-like ME than from o/w ME although the respective release rates across artificial membranes were the same (Tables IV and VI). This phenomenon could be explained by lower amount of penetration enhancer isopropyl myristate in gel-like ME. o/w ME carbomer delivered the lowest amount of vitamin C across the skin (p = 0.002 and p < 0.001 compared to gel-like and o/w ME, respectively) and its permeation rate was also the lowest.
Table VI.
Steady-State Flux (J) of Vitamin C Through Pig Ear Skin from Different ME at 32°C
| Formulation | J (nmol/(h cm2)) |
|---|---|
| o/w ME | 0.382 ± 0.049 |
| gel-like ME | 0.286 ± 0.053* |
| o/w ME carbomer | 0.076 ± 0.015* |
*p < 0.05 compared to o/w ME
Cell Cytotoxicity
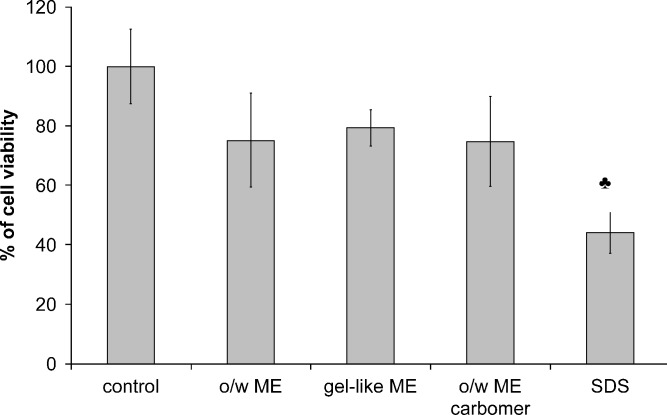
Figure 5 summarizes the cell viability after 24-h exposure of HEK293 cells to different ME that contained no vitamin. HEK293 cells were chosen for cytotoxicity experiments since they are, like normal human keratinocytes, epithelial adherent, but they allow simple manipulation and suitable visualization. Moreover, this cell culture has already been used to study the photoprotective effects of antioxidants in liposomes (21).
Fig. 5.
Cytotoxicity of gel-like ME, o/w ME, and o/w ME carbomer according to MTT assay. SDS was used as a positive control. ♣ p < 0.05 compared to control
All ME show good cell viabilities (larger than 75%). No significant difference (p < 0.05) among formulations was seen, which was expected as all systems contained approximately the same amount of surfactants, the components of ME that are usually associated with irritation. All tested ME performed considerably better (p < 0.001) than the positive control (SDS solution in the same final concentration as ME), a standard irritant in cell culture models. The results indicate that by selecting pharmaceutically acceptable ingredients, it is possible to produce low toxicity ME.
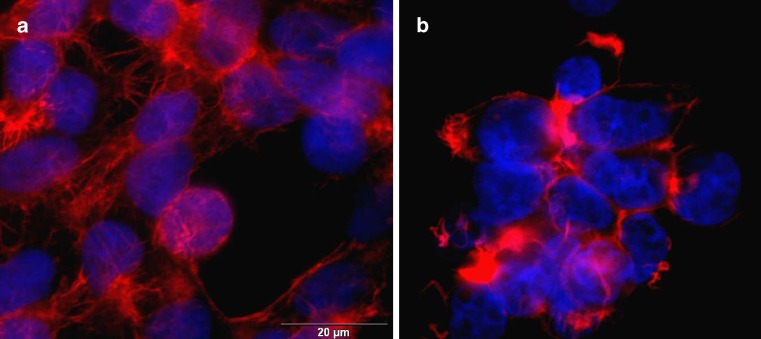
The effect of ME on cell morphology was examined by fluorescence microscopy. On Fig. 6, representative pictures of control cells and cells treated with ME are shown. As no difference among different ME was observed, only a picture of cell culture treated with o/w ME is represented. No difference in morphology of cell nuclei was observed. However, changes in cytoskeletal architecture reflected in different organization of actin fibers that could have as a consequence changes in membrane integrity has been observed, probably due to a presence of surfactant molecules. This changes in cell morphology helps to explain the ≈25% loss of cell viability in cell culture after the exposure to ME.
Fig. 6.
Fluorescent transmission micrographs of a control HEK293 cells and b HEK293 cells treated with o/w ME
CONCLUSION
Microemulsion gel with temperature-sensitive rheological behavior has been proved an effective and non-irritant vehicle with functionally suitable consistency for simultaneous topical delivery of a hydrophilic vitamin C and a lipophilic vitamin E.
Acknowledgment
Authors would like to thank Prof. Hans E. Junginger for helpful discussion. We are grateful to assistant Karmen Teskač for her assistance with fluorescence microscope.
Footnotes
This work was supported by a grant of Slovenian Research Agency.
References
- 1.Kreilgaard M. Influence of microemulsions on cutaneous drug delivery. Adv. Drug Del. Rev. 2002;54(Suppl 1):S77–S98. doi: 10.1016/S0169-409X(02)00116-3. [DOI] [PubMed] [Google Scholar]
- 2.Welin-Berger K., Neelissen J., Bergenstahl B. In vitro permeation profile of a local anaesthetic compound from topical formulations with different rheological behaviour-verified by in vivo efficacy data. Eur. J. Pharm. Sci. 2001;14:229–236. doi: 10.1016/S0928-0987(01)00181-6. [DOI] [PubMed] [Google Scholar]
- 3.Libster D., Aserin A., Garti N. A novel dispersion method comprising a nucleating agent solubilized in a microemulsion, in polymeric matrix I. Dispersion method and polymer characterization. J. Colloid Interface Sci. 2006;299:172–181. doi: 10.1016/j.jcis.2006.01.064. [DOI] [PubMed] [Google Scholar]
- 4.Eccleston G. M. Microemulsions. In: Swarbirck J., Boylan J. C., editors. Encyclopedia of Pharmaceutical Technology. New York: Dekker; 1994. pp. 411–412. [Google Scholar]
- 5.Spiclin P., Homar M., Zupancic-Valant A., Gasperlin M. Sodium ascorbyl phosphate in topical microemulsions. Int. J. Pharm. 2003;256:65–73. doi: 10.1016/S0378-5173(03)00063-2. [DOI] [PubMed] [Google Scholar]
- 6.Adhami V. M., Syed D. N., Khan N., Afaq F. Phytochemicals for prevention of solar ultraviolet radiation-induced damages. Photochem. Photobiol. 2008;84:489–500. doi: 10.1111/j.1751-1097.2007.00293.x. [DOI] [PubMed] [Google Scholar]
- 7.Svobodova A., Walterova D., Vostalova J. Ultraviolet light induced alteration to the skin. Biomed. Pap. Med. Fac. Palacky Univ. Olomouc Czech Repub. 2006;150:25–38. doi: 10.5507/bp.2006.003. [DOI] [PubMed] [Google Scholar]
- 8.Burke K. E. Interaction of vitamins C and E as better cosmeceuticals. Dermatol. Ther. 2007;20:314–321. doi: 10.1111/j.1529-8019.2007.00145.x. [DOI] [PubMed] [Google Scholar]
- 9.Lupo M. P. Antioxidants and vitamins in cosmetics. Clin. Dermatol. 2001;19:467–473. doi: 10.1016/S0738-081X(01)00188-2. [DOI] [PubMed] [Google Scholar]
- 10.Lin J. Y., Selim M. A., Shea C. R., Grichnik J. M., Omar M. M., Monteiro-Riviere N. A., Pinnell S. R. UV photoprotection by combination topical antioxidants vitamin C and vitamin E. J. Am. Acad. Dermatol. 2003;48:866–874. doi: 10.1067/mjd.2003.425. [DOI] [PubMed] [Google Scholar]
- 11.Niki E., Noguchi N., Tsuchihashi H., Gotoh N. Interaction among vitamin C, vitamin E, and beta-carotene. Am. J. Clin. Nutr. 1995;62:1322S–1326S. doi: 10.1093/ajcn/62.6.1322S. [DOI] [PubMed] [Google Scholar]
- 12.Spernath A., Aserin A. Microemulsions as carriers for drugs and nutraceuticals. Adv. Colloid Interface Sci. 2006;128–130:47–64. doi: 10.1016/j.cis.2006.11.016. [DOI] [PubMed] [Google Scholar]
- 13.Kaur I. P., Kapila M., Agrawal R. Role of novel delivery systems in developing topical antioxidants as therapeutics to combat photoageing. Ageing Res. Rev. 2007;6:271–288. doi: 10.1016/j.arr.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 14.Bonacucina G., Cespi M., Misici-Falzi M., Palmieri G. F. Colloidal soft matter as drug delivery system. J. Pharm. Sci. 2008;98(1):1–42. doi: 10.1002/jps.21423. [DOI] [PubMed] [Google Scholar]
- 15.Kristl J., Teskac K., Milek M., Mlinaric-Rascan I. Surface active stabilizer Tyloxapol in colloidal dispersions exerts cytostatic effects and apoptotic dismissal of cells. Toxicol. Appl. Pharmacol. 2008;232(2):218–225. doi: 10.1016/j.taap.2008.06.019. [DOI] [PubMed] [Google Scholar]
- 16.Tomsic M., Podlogar F., Gasperlin M., Bester-Rogac M., Jamnik A. Water-Tween 40/Imwitor 308-isopropyl myristate microemulsions as delivery systems for ketoprofen: small-angle X-ray scattering study. Int. J. Pharm. 2006;327:170–177. doi: 10.1016/j.ijpharm.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 17.Podlogar F., Bester Rogac M., Gasperlin M. The effect of internal structure of selected water-Tween 40-Imwitor 308-IPM microemulsions on ketoprofene release. Int. J. Pharm. 2005;302:68–77. doi: 10.1016/j.ijpharm.2005.06.023. [DOI] [PubMed] [Google Scholar]
- 18.Koleng J. J., McGinity J. W., Wilber W. R. Carbomer. In: Rowe R. C., Sheskey P. J., Weller P. J., editors. Handbook of Pharmaceutical Excipients. London: Pharmaceutical; 2003. pp. 89–91. [Google Scholar]
- 19.Lee A. R., Tojo K. An experimental approach to study the binding properties of vitamin E (alpha-tocopherol) during hairless mouse skin permeation. Chem. Pharm. Bull. 2001;49:659–663. doi: 10.1248/cpb.49.659. [DOI] [PubMed] [Google Scholar]
- 20.Guo H., Liu Z., Li J., Nie S., Pan W. Effects of isopropyl palmitate on the skin permeation of drugs. Biol. Pharm. Bull. 2006;29:2324–2326. doi: 10.1248/bpb.29.2324. [DOI] [PubMed] [Google Scholar]
- 21.Caddeo C., Teskac K., Sinico C., Kristl J. Effect of resveratrol incorporated in liposomes on proliferation and UV-B protection of cells. Int. J. Pharm. 2008;363(1–2):183–191. doi: 10.1016/j.ijpharm.2008.07.024. [DOI] [PubMed] [Google Scholar]