Abstract
This study was designed for investigating the effect of Asparagus racemosus (AR) extract and chitosan (CTN) in facilitating the permeation of carvedilol (CDL) across rat epidermis. Transdermal flux of carvedilol through heat-separated rat epidermis was investigated in vitro using vertical Keshary–Chien diffusion cells. Biophysical and microscopic manifestations of epidermis treated with AR extract, CTN, and AR extract–CTN mixture were investigated by using differential scanning calorimetry, transepidermal water loss, scanning electron microscopy (SEM), and transmission electron microscopy (TEM). Biochemical estimations of cholesterol, sphingosine, and triglycerides were carried out for treated excised as well as viable rat epidermis. The antihypertensive activity of the patches in comparison with that of oral carvedilol was studied in deoxycorticosterone acetate-induced hypertensive rats. The permeation of carvedilol across excised rat epidermis was significantly higher (p < 0.05) when AR extract, CTN, or AR extract–CTN mixture was used as donor vehicle as compared to propylene glycol/ethanol (7:3) mixture. Epidermis obtained after 12 h treatment of viable rat skin with AR extract–CTN mixture showed significantly higher (p < 0.05) permeability to CDL as compared to that after treatment with AR extract or CTN alone. Further, the application of patches containing AR extract–CTN mixture resulted in sustained release of CDL which was able to control the hypertension in deoxycorticosterone acetate-induced hypertensive rats through 36 h. Estimation of micro constituents in rat epidermis revealed maximum extraction of cholesterol, sphingosine, and triglycerides after treatment with AR extract–CTN mixture. This was manifested in altered lipid and protein-specific thermotropic transitions. Further, increase in intercellular space, disordered lipid structure, and corneocyte detachment as observed in SEM and TEM suggested great potential of AR extract for use as percutaneous permeation enhancer. The developed transdermal patches of CDL containing AR extract–CTN mixture exhibited better performance as compared to oral administration in controlling hypertension in rats.
Keywords: Asparagus racemosus, biochemical estimation, carvedilol, chitosan, differential scanning calorimetry, saponin, scanning electron microscopy, transmission electron microscopy
INTRODUCTION
The nonviable outermost layer of skin, stratum corneum (SC), serves as a penetration, dehydration, and protection barrier against various environmental hazards. The SC consists of several layers of overlapping corneocytes, embedded in a lipid matrix of ordered lamellae. Despite their overall small percentage, the lipids of SC represent the main barrier to diffusion of substances. These barrier properties are based on the unique composition and organization of the intercellular lipid lamellae (1–3).
Several physical (sonophoresis, iontophoresis, electro-osmosis, electroporation, and temperature), chemical methods, or formulations reported in the literature have been investigated for elevating the amount of drugs delivered across and into the skin. Currently, the most widely used approach for drug permeation enhancement across the SC is the use of chemical penetration enhancers (sorption promoters and accelerants). The modes of action of these agents have been reviewed in detail by several authors (4,5). These enhancers are reported to exert multiple effects such as increasing the diffusivity of the drug in the skin, causing SC lipid-fluidization, decreasing the barrier function (a reversible action), and enhancing the thermodynamic activity of the drug in the vehicle and skin. This results in a reservoir of drug within the skin, affecting the partition coefficient of the drug, which ultimately results in release of drug from the formulation into the upper layers of the skin (6).
Transdermal formulations often employ surfactants to aid permeation of drug molecules across the SC. However, safety concerns related to transport into systemic circulation as well as long time required for reversibility of effect are shifting the impetus from synthetic to natural surfactants. Two primary considerations arise while using surfactants for enhancing drug transport across biological membranes. The first consideration relates to temporary alteration of integrity of the rate-limiting barrier, which can be manifested in the form of increased membrane fluidity, solubilization, and/or extraction of lipids present in biological membranes and alterations in the tight junction properties (7–9). The second consideration entails the interaction of drug with surfactants. These physicochemical interactions manifest themselves in terms of enhanced solubility and/or dissolution of drug, prevention of drug precipitation if administered in solution form, and reduction in drug activity. The increase/reduction in drug activity is a consequence of interaction of drug with aggregates and/or micelles formed by the surfactant (10). Saponins constitute a vast group of glycosides occurring in plants. They are characterized by their surface active properties and, hence, possess great potential for use as percutaneous permeation enhancer. A large number of biological effects of saponins have been ascribed to their action on membranes. In fact, the specific ability to form pores in membranes has contributed to their widespread use in physiological research (11–13).
It has been found that, while the perforations caused by substances such as vitamin A were reversible in nature, the membrane pores or defects produced by saponins were long-lasting, and these membranes were permeable to large molecules like ferritin for longer period (14). The lesions caused by saponins are thought to be due to micelle-like aggregations between them and cholesterol in the plane of the membrane, possibly with saponin molecules arranged in a ring with their hydrophobic moieties combined with cholesterol around the outer perimeter (15). Other reports depict the interactions between saponins and biological membranes to be more complex. Brain et al. (16) showed that insertion of the aglycone into the lipid bilayer is independent of the presence of cholesterol. Saponins may interact with the polar heads of membrane phospholipids and the –OH group of cholesterol through –OH groups at C3 or C28, which results in the ability of the later to form micelle-like aggregates. Moreover, their hydrophobic aglycone backbone could intercalate into the hydrophobic interior of the bilayer. Both of these effects may contribute to the alteration of the lipid environment around membrane proteins. Asparagus racemosus (AR) extract contains steroidal saponins. The interaction of these saponins with the skin membrane lipids can be expected to enhance the permeation of drugs from transdermal formulations.
Chitosan (CTN) is a polysaccharide and carries high positive charge when dissolved in organic acids. It is reported to open the tight junctions in the intestinal epithelium (17). This property of CTN was observed to be responsible for enhanced permeability of apical membrane and tight junctions. Tight junctions are also present in the skin, and CTN is reported to possess the ability to open these tight junctions in the skin (18). Hence, CTN can be envisaged to alter the organization of the ordered skin lipids thereby, allowing enhanced percutaneous permeation of drugs.
Some enhancers cause permanent epidermal damage that can only be repaired by SC regeneration (3). However, for other enhancers, the increased permeability of SC may return to its normal state after their removal from the site of application. This temporary effect is attributed to the transient interactions between the enhancers and SC lipids, which is the major diffusion passage for almost all small chemicals (19). In the light of these reports, the use of AR extract containing steroidal saponins and CTN in transdermal formulations can be envisaged to offer a facile approach for enhancing the percutaneous permeation of drugs.
Carvedilol (CDL) is a β-adrenoreceptor antagonist with α1-adrenoreceptor antagonistic activity widely used for the treatment of hypertension. The dosage forms of CDL presently approved by US Food and Drug Administration available for clinical use include tablets and carvedilol phosphate as extended release capsules. These tablets are needed to be administered two times daily for effective control of hypertension in patients due to its short biological half-life of 2.2 h and very low oral availability of 25% due to extensive first-pass metabolism (20). CDL possesses ideal characteristics of low molecular weight (406.5), favorable logarithmic partition coefficient (log octanol/water, 0.58 ± 0.02; log octanol/buffer pH 7.4, 0.61 ± 0.06), and small dose range (25–50 mg) making it suitable for transdermal delivery. These facts coupled with the requirement of maintaining constant plasma drug concentration above minimum effective concentration over long duration for effective control of hypertension makes transdermal route an attractive mode of delivery for CDL. This route of administration has not yet been exploited for CDL.
The present investigation was therefore aimed at evaluating the influence of saponin-containing AR extract and its combination with CTN on in vitro and in vivo permeation of CDL across rat skin. Pharmacodynamic evaluation of the formulation was done in deoxycorticosterone acetate (DOCA)-induced hypertensive rats.
MATERIALS AND METHODS
Methods
Extraction Procedure
The ground roots were defatted in light petroleum (b.p. = 40–60°) in a Soxhlet apparatus. The residue was then dried at room temperature. The defatted roots underwent a second extraction in absolute ethanol in a soxhlet apparatus for 48 hr until colorless extract was obtained. The ethanolic extract was then dried under vacuum in a rotary evaporator (model no. 954, Perfit-Gupta Scientific Industries, Ambala Cantt, India). The residue obtained was dissolved in water, and then it was further extracted four times with n-butanol. The butanolic extracts were combined and evaporated to dryness to obtain a mixture of saponins.
Preparation of Epidermal Sheets
Epidermal sheets were obtained from the whole skin of Albino Wistar rats of either sex (175–225 g). The procedure for separating epidermis from whole thickness skin as described earlier was used (21). Freshly separated epidermal sheets were used in all the experiments.
Determination of Critical Micelle Concentration of AR Extract and its Effect on Solubility and Partition Coefficient of CDL
AR extract (0.5–10% w/v) was dissolved in a mixture of propylene glycol/ethanol (7:3). The surface tension of each solution was determined using stalagmometer (Model no. 694-A, Perfit-Gupta Scientific Industries). Each experiment was performed in triplicate. Log concentration of AR extract was plotted against surface tension (dynes/cm) obtained by employing the respective CDL solutions. The concentration at which no further decrease in surface tension was observable was considered as the critical micelle concentration (CMC) of AR extract.
The solubility of carvedilol in phosphate buffer (PB) or in PB solutions containing different concentrations of AR extract was determined. Excess CDL was added and stirred at 37 ± 2°C in shaker incubator (C-24, Remi Equipment, Mumbai) for 24 h. The solutions were filtered through G-4 filter, and the filtrates were immediately analyzed for CDL spectrofluorimetrically employing excitation and emission wavelength of, respectively, 282 and 350 nm. Each experiment was performed in triplicate.
The partition coefficient (KIPM/PB) was determined by adding CDL (50 mg) to a 1:4 saturated mixture of isopropyl myristate (IPM) and PB containing PEG 400 (10% w/v) in absence or presence of different concentrations of saponins. The mixture was stirred at 37 ± 2°C in shaker incubator for 24 h. The concentration of CDL in PB was determined spectrofluorimetrically employing excitation and emission wavelength of 282 and 350 nm, respectively. AR extract could not be employed beyond 0.5% w/v for determination of solubility and partition coefficient because color of the extract was found to interfere with estimation of drug.
Influence of Different Donor Formulations on In Vitro Permeation of CDL
Freshly obtained rat epidermal sheets were mounted on a vertical Keshary–Chien diffusion cell. CDL dispersed in 4 ml of propylene glycol/ethanol (7:3) mixture was mixed with different concentrations of AR extract, CTN (1% w/v), or solution containing mixture of AR extract and CTN (1% w/v) and loaded in the donor compartment. The donor compartment was sealed using a parafilm. The receptor fluid containing phosphate buffer pH 7.4 (PB), sodium azide (0.05% w/v), and PEG 400 (10% w/v) was maintained at 32 ± 1°C and stirred at 30 rpm. The receptor fluid samples (1 ml) were withdrawn repeatedly through 48 h and analyzed using spectrofluorometer (Model No. SL-174, Elico Pvt. Ltd., Hyderabad) employing excitation and emission wavelengths of 282 and 350 nm, respectively. An equal volume of fresh receptor fluid was replenished in the receptor compartment after each sampling.
In Vitro Permeation of CDL Across AR Extract-Treated Viable Rat Epidermis
Two patches (∼7 cm2), one on either side of spinal cord, were prepared by shaving with an electric razor and left undisturbed for 24 h. One patch was left untreated and served as control. The other patch received application of AR extract (1% w/v), CTN (1% w/v), or solution containing mixture of AR extract and CTN. The animals were killed after 4, 12, 24, or 48 h of application. Epidermal sheets obtained from these excised patches were used for studying the in vitro permeation of CDL using vertical Keshary–Chien diffusion cells as described earlier.
Influence of Different Treatment on Cholesterol, Triglycerides, and Sphingosine Content in Excised and Viable Rat Epidermis
Freshly excised rat epidermal sheets were treated with AR extract (1% w/v), CTN (1% w/v), or AR extract–CTN mixture for 48 h. Transdermal patches containing these formulations were prepared using adhesive tape, polyethylene backing membrane, and a rubber ring. They were applied to shaved skin on the dorsal portion of rats. The animals were killed after 4, 12, 24, or 48 h, and epidermis was separated from the whole skin patches. All epidermal sheets were dried to constant weight at 50°C, and total lipids were extracted by Folch method (22). Cholesterol (CHOL) and triglyceride (TGS) content in these extracts was determined by using respective diagnostic kit. Sphingosine (SGE) content was determined spectroflourometrically (SL-174 spectrofluorimeter, Elico limited, India) using excitation and emission wavelengths of 340 and 455 nm, respectively, according to the method outlined by Sabbadini et al. (23).
Differential Scanning Calorimetric Analysis
Differential scanning calorimetric (DSC) analysis (20°C to 120°C, 1°C/min) was carried out on excised untreated rat epidermal sheets as well as those obtained after treatment with different concentrations of AR extract, CTN (1% w/v) or AR extract–CTN mixture (Mettler Toledo Star System, 821 E, Switzerland). Aluminum pans of 40 μl capacity with hermetically sealed aluminum lid were used for the DSC analysis. Epidermal sheets were washed with distilled water thoroughly and were dried. Samples of dried epidermal sheets were hydrated over a saturated sodium chloride solution (75% relative humidity at 25°C) for 3 days prior to DSC analysis.
Scanning Electron Microscopy and Transmission Electron Microscopy
Whole thickness skin samples were removed following 4, 12, 24, or 48 h application of AR extract (1%), CTN (1%), or AR extract–CTN mixture to viable rat skin and also after 48 h treatment to excised skin. The epidermal sheets were separated from the whole thickness skin samples and fixed for 4 h at 4°C in gluteraldehyde (2.5% w/v) in 0.1 M cacodylate buffer (pH 7.2) for scanning electron microscopy (SEM). The samples were washed three times in 0.1 M cacodylate buffer (pH 7.2) for 10 min each to remove excess fixative. Post fixation was performed for 1 h at 4°C in 1% w/v osmium tetraoxide solution in 0.1 M cacodylate buffer (pH 7.2). After rinsing two times in buffer, these specimens were dehydrated by placing in graded ethanol solutions and critically dried in a critical point drier. The samples were then mounted on clean aluminum stubs with silver PAG-915 and coated with gold palladium alloy (160 Å thickness) on a sputter coater (24). Specimens were then observed under scanning electron microscope (LEO 435VP) and photographed.
Transmission electron microscopy (TEM) investigations were carried out for visualizing the change in ultra structure of epidermis after different treatments. Epidermal sheets separated from excised whole thickness skin samples after 48 h treatment with selected concentrations of AR extract, CTN (1% w/v), or mixture of CTN (1% w/v) and selected concentration of AR extract were fixed in Karnovosky’s fixative {paraformaldehyde (2% w/v) and glutaraldehyde (1% w/v) in 0.1 M phosphate buffer (pH 7.4)} and processed (25). Ultra-thin (60–90°Å) sections were cut and double stained with uranyl acetate and lead citrate for TEM observation (Morgani-268). The process was also carried out for epidermal sheets obtained from viable skin treated with the same formulations and excised after 4, 12, 24, or 48 h.
Transepidermal Water Loss Studies
Two patches (∼7 cm2), one on either side of spinal cord, were prepared by shaving with an electric razor and left undisturbed for 24 h. One patch was left untreated and served as control. The other patch received application of AR extract (1% w/v), CTN (1% w/v), or solution containing mixture of AR extract and CTN. The control and treatment sites were marked as circular area (∼7 cm2) with a felt-tip marker on the dorsal portion of the rat. The patches were removed after 4, 12, 24, or 48 h of application. The rats were anaesthetized using anesthetic ether at the time of measuring the transepidermal water loss (TEWL). Measurements of TEWL were taken after 0, 1, 2, 4, 6, 12, 20, 24, 36, or 48 h of application and after 48 h of removal of the patch. The TEWL was measured using Tewameter TM 210 (Courage and Khazaka Electronic GmbH, Koln, Germany). The probe of the Tewameter was placed perpendicular to the surface of the skin, and a stable reading of TEWL was reached in about 60 s. The results were expressed in g/hm2. The measurements were performed in an acclimatized room with a mean relative humidity of 50.2 ± 6.9% and a mean room temperature of 21.6 ± 0.6°C as described earlier (26).
Efficacy of Transdermal Patches in DOCA-Induced Hypertensive Rats
Male Wistar rats (150–160 g) of 6–8 weeks age were used in the study. They were fed with standard laboratory chow diet and given water ad libitum before initiation of the study and were exposed to 12 h light and 12 h dark cycle. The experimental protocol was approved by Institutional Animal Ethics Committee of Punjabi University, Patiala, India, and the care and handling of the animals were in accordance with the National Institutes of Health guidelines.
Acclimatization and preparation of the animals was done by following the procedure described by Khazaei and Nematbaksh (27). The blood pressure (BP) was measured by tail-cuff method using non-invasive blood pressure monitoring system (model 229IITC, USA). Hypertension was induced by administering DOCA (30 mg kg−1, s.c.) twice weekly for 5 weeks (27). The animals selected after 4 weeks of DOCA administration were divided into three groups (n = 6). Group I served as control, group II received CDL 10 mg/kg orally, group III received application of transdermal patch containing AR extract–CTN mixture. BP was measured at different time intervals (0, 1, 2, 4, 6, 8, 20, 22, 24, 30, 36 or 48 h).
Evaluation of Pharmacokinetic Parameters of Carvedilol in Animals
Wistar albino rats (200–250 g) were used. A patch of ∼7 cm2 was prepared on the dorsal side by shaving with electric shaver and left untreated for 24 h. The rats were divided into three groups (n = 6). Group I was administered CDL orally (10 mg/kg as a 0.5% carboxymethyl cellulose suspension), group II received transdermal patch of CDL (40 mg) dispersed in propylene glycol/isopropyl alcohol (PG/ETOH; 7:3), and group III received transdermal patch of CDL (40 mg) containing AR extract–CTN mixture. The blood samples were withdrawn at different time intervals (1, 2, 4, 6, 8, 10, 12, 24, 28, 36, or 48 h) and analyzed by high-performance liquid chromatography (HPLC; Water’s HPLC System with Waters 600 controller pump, Waters inline degasser AF, Photodiode Array Detector-2996), C8 spherisorb column (4.6 mm × 250 mm) 5μ and Empower Pro software) at 242 nm. The protocol was approved by Institutional Animal Ethics Committee of Punjabi University, Patiala, India. The drug concentration in the plasma was measured. The pharmacokinetic parameters of CDL were estimated by using WinNonlin version 5.2 (Pharsight, Mountain View, CA, USA).
Statistical Analysis
Analysis of variance followed by Duncan or Dunnett test was used for statistical comparison of the data. Significance level was fixed at 0.05.
RESULTS
The surface tension increased from 30.05 to 31.25 when the concentration of AR extract was increased from 0.5% to 1%, respectively. However, an increase in concentration of AR extract beyond 1% w/v did not result in further increase of surface tension indicating 1% w/v concentration to be the CMC of AR extract. The solubility of CDL in PB was observed to increase with increase in concentration (0.1–0.5% w/v) of AR extract. The enhancement of aqueous solubility of CDL was associated with decrease in partition coefficient (KIPM/PB) of CDL.
The data summarized in Table I shows that the permeation of CDL using AR extract, CTN, or AR extract–CTN mixture as donor formulations across excised rat epidermis was significantly higher (p < 0.05) as compared to that using propylene glycol–ethanol (7:3) mixture. Maximum flux of CDL (157.69 μg/cm2/hr) was observed when AR extract–CTN mixture was used as donor vehicle. Similarly, the permeation of CDL across epidermis excised from viable skin after treatment for 12 h with AR extract–CTN mixture was observed to be significantly greater (p < 0.05) as compared to that obtained across epidermis treated with either AR extract or CTN alone (Table II). Further, comparison of Tables I and II indicates significantly greater (p < 0.05) permeation of CDL across excised epidermis as compared to that across epidermis excised from viable skin after any treatment.
Table I.
In Vitro Permeation of CDL from Different Formulations Across Excised Rat Epidermis
| Treatment | Flux (μg/cm2/hr) | Enhancement Ratio (ER) |
|---|---|---|
| Donor Formulation PG/ETOH (7:3) | 22.79 ± 2.34 | – |
| Buffer (pH 6) | 20.6 ± 1.81 | – |
| AR (0.5% w/v) | 34.78 ± 3.08 | 1.53 |
| AR (1% w/v) | 121.61 ± 5.39 | 5.34 |
| AR (2.5% w/v) | 98.36 ± 4.62 | 4.32 |
| AR (5% w/v) | 70.59 ± 3.88 | 3.09 |
| AR (10% w/v) | 62.39 ± 4.15 | 2.74 |
| CTN (1% w/v) | 103.5 ± 5.19 | 4.54 |
| CTN (1% w/v) + AR (1% w/v) | 157.69 ± 8.46 | 6.92 |
PG propylene glycol, ETOH ethanol, CTN chitosan, AR A. racemosus extract dissolved in PG/ETOH mixture
Table II.
In Vitro Permeation of CDL Across Epidermis Excised after Treatment of Viable Rat Epidermis
| Formulation | Excision time (h) | Flux (μg/cm2/h) | Enhancement ratio (ER) |
|---|---|---|---|
| AR | 4 | 25.17 ± 1.88 | 1.10 |
| 12 | 80.75 ± 4.94 | 3.54 | |
| 24 | 65.59 ± 4.02 | 2.88 | |
| 48 | 44.41 ± 2.46 | 1.95 | |
| CTN | 4 | 24.94 ± 1.05 | 1.09 |
| 12 | 60.13 ± 3.68 | 2.64 | |
| 24 | 33.28 ± 2.82 | 1.46 | |
| 48 | 30.29 ± 2.91 | 1.33 | |
| AR + CTN | 4 | 79.69 ± 4.35 | 3.36 |
| 12 | 91.65 ± 5.08 | 4.02 | |
| 24 | 64.51 ± 3.98 | 2.83 | |
| 48 | 50.90 ± 2.88 | 2.23 |
The quantity of CHOL, TGS, or SGE extracted from excised epidermis increased and was maximum for epidermis treated for 48 h with AR extract (1% w/v). The treatment with CTN (1% w/v) was not as effective as AR extract in perturbing microconstituents because the quantity of CHOL, TGS, or SGE extracted was significantly less (p < 0.05) as compared to that extracted by AR extract. However, treatment with combination of CTN and AR extract produced maximum perturbation as evident from highest quantities of cholesterol (69.25%), triglyceride (49.11%), and sphingosine (35.99%) extracted from excised epidermis. It is interesting to note that all these treatments were significantly less effective (p < 0.05) when applied to viable skin. Further, the maximum effect in viable skin was observed when epidermis was excised after 12 h of application of any formulation (Table III).
Table III.
Effect of Treatment with A. racemosus Extract, Chitosan, or Their Mixture on Microconstituents of Epidermis in Excised and Viable Rat Skin
| Formulation | Skin microconstituent extracted (%) | ||
|---|---|---|---|
| Cholesterol | Triglyceride | Sphingosine | |
| Skin type: excised | |||
| AR (0.5%) | 18.198 ± 2.01 | 6.59 ± 0.59 | 5.863 ± 0.851 |
| AR (1%) | 22.05 ± 2.65 | 16.54 ± 2.06 | 13.578 ± 1.87 |
| AR (2.5%) | 38.58 ± 3.94 | 21.681 ± 3.99 | 19.962 ± 2.06 |
| AR (5%) | 43.585 ± 4.68 | 36.6 ± 4.28 | 25.731 ± 2.64 |
| AR (10%) | 57.732 ± 6.13 | 38.35 ± 3.64 | 29.457 ± 3.58 |
| CTN (1%) | 19.120 ± 2.17 | 6.019 ± 0.58 | 7.346 ± 0.58 |
| CTN (1%) + AR (1%) | 69.251 ± 4.35 | 49.11 ± 2.16 | 35.99 ± 1.97 |
| Skin type: viable | |||
| CTN (1% w/v) | |||
| 4 h | 1.711 ± 0.98 | 1.91 ± 0.25 | 0.6872 ± 0.27 |
| 12 h | 7.105 ± 1.108 | 12.001 ± 2.01 | 2.4023 ± 0.077 |
| 24 h | 2.526 ± 0.99 | 0.501 ± 0.11 | 0.28685 ± 0.061 |
| 48 h | 2.011 ± 0.839 | 0.489 ± 0.884 | 0.2015 ± 0.624 |
| AR (1% w/v) | |||
| 4 h | 10.831 ± 0.91 | 8.993 ± 0.73 | 6.5016 ± 0.21 |
| 12 h | 21.33 ± 2.34 | 14.974 ± 1.68 | 11.25 ± 1.32 |
| 24 h | 12.33 ± 1.432 | 5.392 ± 0.66 | 5.61 ± 0.68 |
| 48 h | 6.665 ± 1.24 | 2.186 ± 0.69 | 1.008 ± 0.57 |
| AR (1%) + CTN (1%) | |||
| 4 h | 14.263 ± 0.69 | 12.314 ± 0.68 | 8.011 ± 0.48 |
| 12 h | 30.128 ± 3.14 | 17.124 ± 2.03 | 15.198 ± 1.99 |
| 24 h | 13.433 ± 1.58 | 7.013 ± 0.99 | 5.294 ± 0.94 |
| 48 h | 7.125 ± 1.22 | 2.884 ± 0.28 | 2.997 ± 0.35 |
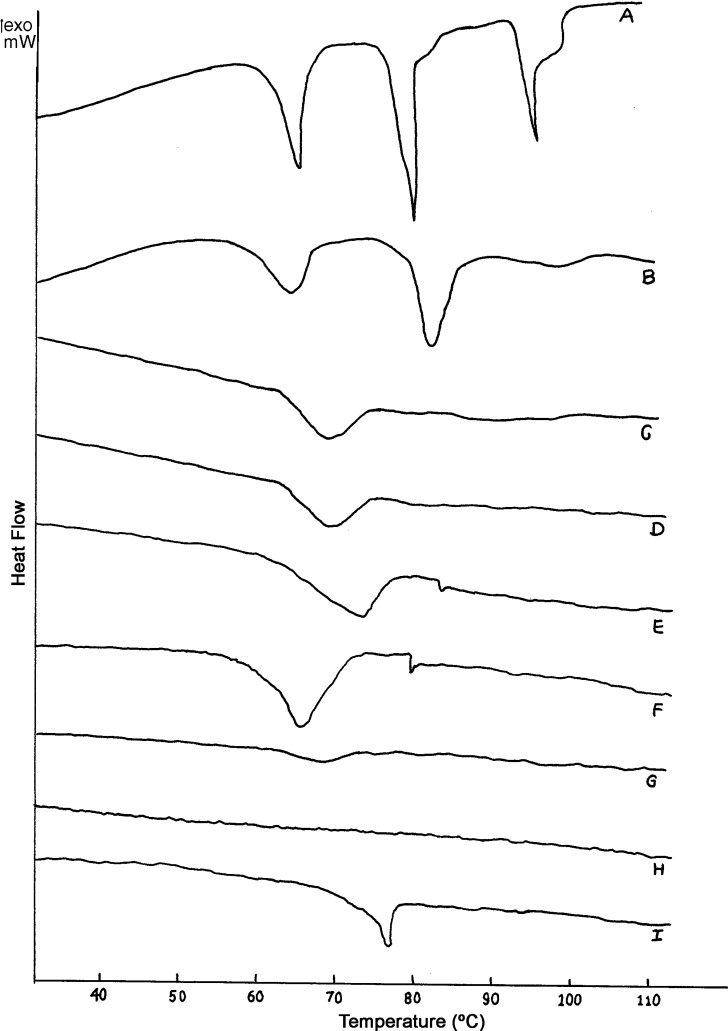
Figure 1 depicts the DSC thermogram of rat epidermis after treatment with AR extract. The normal (untreated) epidermis (Fig. 1 A) exhibited three endothermic transitions each at 70°C (T2), 80°C (T3), and 97°C (T4). Treatment with PG/ETOH (7:3) mixture obliterated the T4 endotherm. The T4 endotherm was also absent in thermograms of excised epidermis treated with 0.5–10% w/v aqueous solutions of AR extract (Fig. 1 B, C). Further, treatment with AR extract–CTN mixture obliterated all the endothermic transitions (Fig. 1 H).
Fig. 1.
DSC thermograms of normal rat epidermis (A) and after treatment with PG/ETOH 7:3 (B), AR 0.5% w/v (C), AR 1% w/v (D); AR 2.5%w/v (E), AR 5% w/v (F); AR 10% w/v (G); AR–CTN mixture (H); CTN 1% w/v (I)
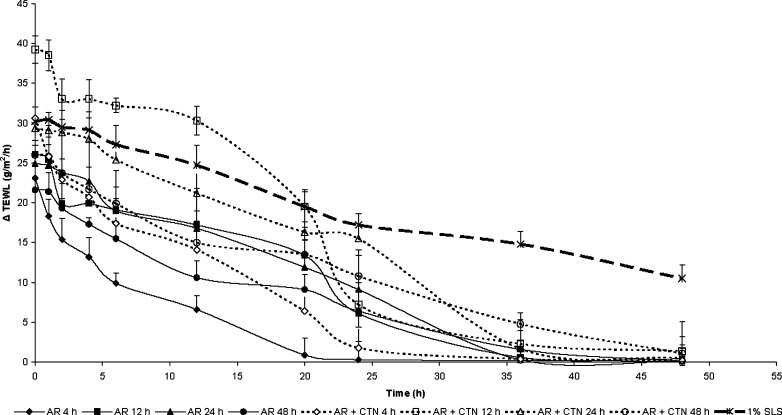
Figure 2 compares the effect of 1% w/v sodium lauryl sulphate (SLS), AR extract (1% w/v), or AR extract (1% w/v)–CTN (1% w/v) mixture on water permeability status of viable rat epidermis. All treatments increased the TEWL significantly as compared to the control. The maximum TEWL was observed after SLS (1% w/v) treatment for 48 h in viable rat epidermis, and the effect did not normalize even after 48 h of removal of this patch. However, after treatment with AR extract or AR extract–CTN mixture, the TEWL decreased to values close to that of the control after 36 h and completely normalized after 48 h.
Fig. 2.
Comparison of effect of treatment of 1% sodium lauryl sulphate with AR (A. racemosus) or AR–CTN (A. racemosus–chitosan) mixture on TEWL of epidermis in viable rat skin after different time intervals
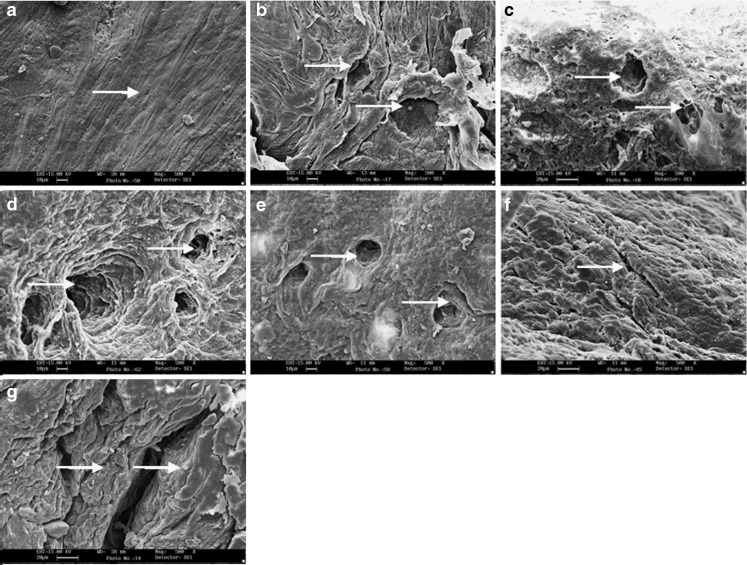
SEM photomicrographs revealed untreated rat epidermis to consist of closely united assembly of squames (Fig. 3a). Treatment with AR extract (1% w/v) produced slight loosening of surface layers and small pores in SC (Fig. 3c). Maximum perturbation due to loosening and creation of pores in upper layers of SC was observed after treatment with combination of AR extract and CTN (Fig. 3d). Figure 3e–g depict the photomicrographs of epidermis excised from skin after 12 h of treatment with these formulations. Amongst these samples, maximum effect was produced by treatment with AR extract–CTN mixture as evidenced by maximum pore formation in upper layers of SC (Fig. 3g).
Fig. 3.
Scanning electron micrograph of excised rat skin: without any treatment a; after treatment with CTN b; after treatment with AR c; after treatment with AR–CTN mixture d; and viable skin excised after 12 h of treatment with AR e; CTN f; AR–CTN mixture g. White solid arrow indicates surface effect on stratum corneum, magnification is ×500, shorter scale bar is 10 μm, and longer scale bar is 20 μm
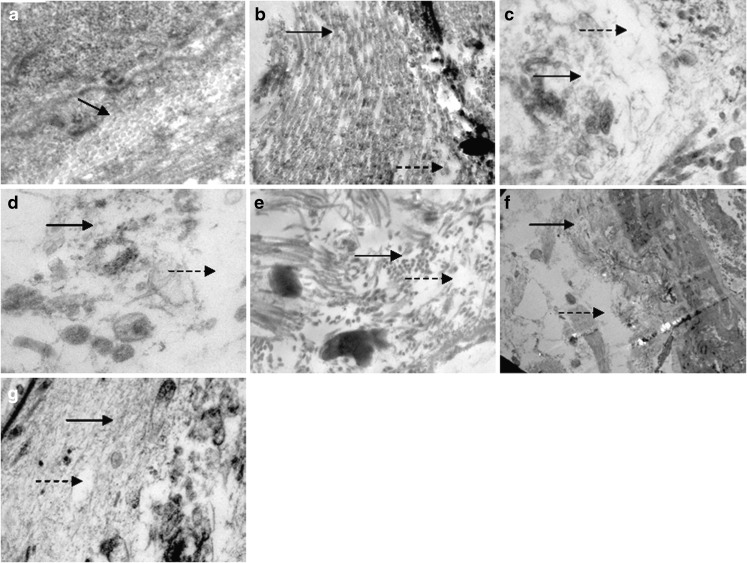
The TEM photomicrographs revealed greater disordering of lipid areas in excised epidermis after treatment with AR extract (Fig. 4c) as compared to CTN treatment (Fig. 4b). The treatment with AR extract–CTN mixture appeared to produce greatest perturbation of excised epidermis as evident from increase in intercellular space, disordered lipid structure, and corneocyte detachment (Fig. 4d). Similar treatments to viable skin for 12 h produced corneocyte detachment and undulation (Fig. 4e–g), where the maximum effect was evident after treatment with AR extract–CTN mixture (Fig. 4g).
Fig. 4.
Transmission electron micrograph of excised rat skin: without any treatment a; after treatment with CTN b; after treatment with AR c; after treatment with AR–CTN mixture d; and viable skin excised after 12 h of treatment with AR e; CTN f; AR–CTN mixture g. Black solid arrows indicate arrangement of corneocytes, and broken arrows indicate lipid perturbation of intercellular lipids, magnification is ×20,000, scale bar — is 100 nm)
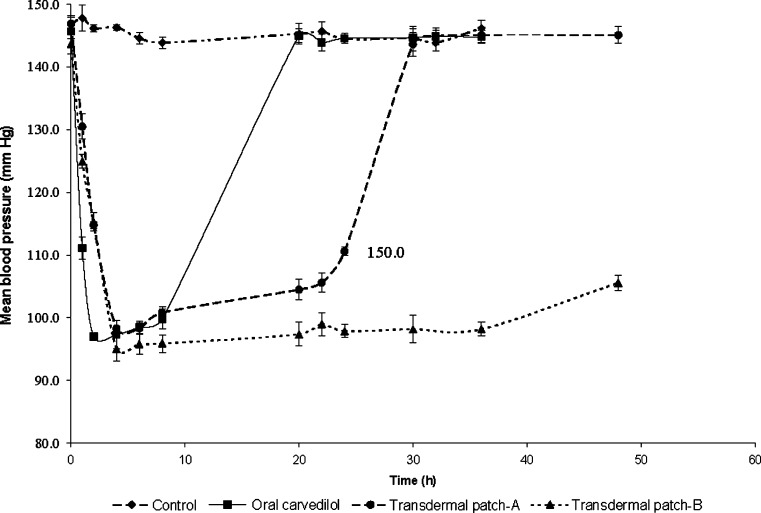
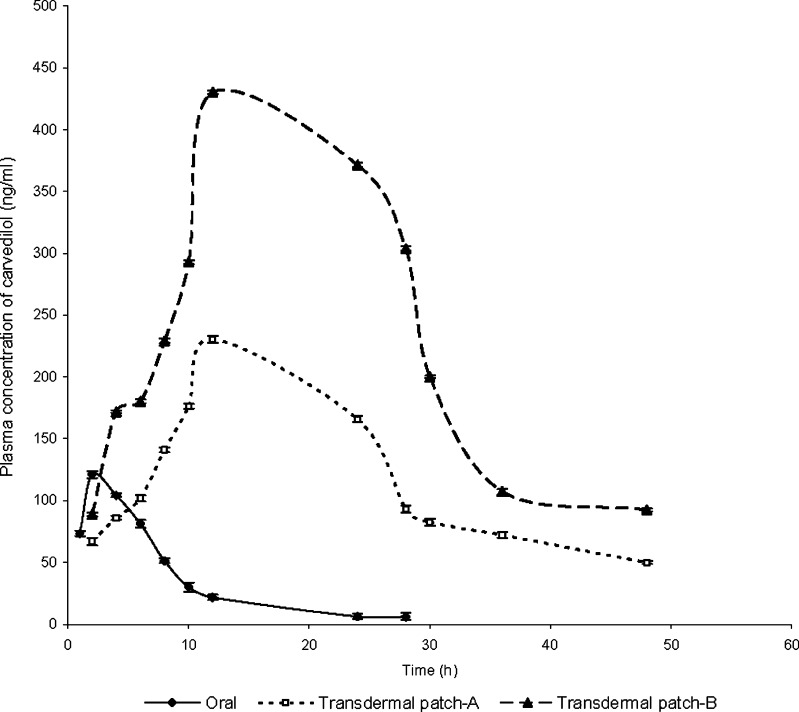
It is evident from Fig. 5 that the administration of DOCA produced significant hypertension in rats. Oral administration of CDL significantly (p < 0.05) controlled the hypertension initially, and the maximum effect was observed after 2 h of administration. However, after 2 h, the BP started rising gradually until it was the same as the initial value at 24 h. In contrast, the BP decreased gradually initially, and maximum reduction was observed at 4 h, which was maintained till 36 h of application of transdermal patches containing AR extract–CTN mixture. The maximum plasma concentration of CDL (Cmax) after oral administration was 121.04 ± 0.98 ng/mL, and Tmax was 2 h. For the transdermal patch containing PG/ETOH (7:3), Cmax and Tmax were 230.33 ± 6.12 ng/mL and 12 h, respectively. However, after application of the transdermal patch containing combination of AR extract and CTN mixture, Cmax and Tmax were 430.06 ± 1.26 ng/mL and 12 h, respectively (Fig. 6).
Fig. 5.
The influence of administration of CDL by oral or transdermal route on blood pressure in DOCA-induced hypertensive rats [Transdermal patch-A: contains PG/ETOH mixture (7:3); Transdermal patch-B: contains AR extract–CTN mixture]
Fig. 6.
Pharmacokinetic profile of CDL following its administration by oral or transdermal route [Transdermal patch-A: contains PG/ETOH mixture (7:3); Transdermal patch-B: contains AR extract–CTN mixture]
DISCUSSION
The surfactant concentration is known to play an important role in enhancing the permeation. The increase in flux at low concentration is normally attributed to the ability of surfactant to penetrate and increase skin permeability. Reduction in rate of transport of drug present in enhancer system is attributed to the ability of surfactant to form micelles, and it is observed only if the interaction between micelle and drug occurs. The observed findings for decreased permeation of carvedilol formulated with greater than 1% w/v concentration of AR extract are in consonance with the findings of Shokri et al. (28), who observed negligible permeation of diazepam in presence of concentrations of Tween 80 greater than its CMC. Similar findings have been reported by other workers (29). Hence, the observed results for carvedilol may be attributed to its greater entrapment into saponins of AR extract micelles when the latter was used at concentrations greater than CMC (1% w/v).
The in vitro permeation of CDL across rat epidermis increased 5.3-fold in the presence of 1% w/v concentration of AR extract. However, no further improvement in CDL permeation was observed on further increasing the concentrations of AR extract (Table I). These observations are in agreement with the report of Nokhodchi et al. (30), who found 10-fold enhancement of in vitro permeation of diclofenac in presence of 0.1% w/v glycyrrhizin (triterpene saponin) to reduce to 1.72-fold in presence of higher (0.5% w/v) concentration of glycyrrhizin.
CTN is reported to open the epidermal tight junctions (18). The effect of CTN on caco-2 monolayer tight junction integrity for antigoat horseradish peroxidase secondary antibodies was found to be maximum at concentration of 0.5%, and translocation of proteins Zo-1 and occluding was completed in 48 h (18). Therefore, it was hypothesized that treatment of viable rat skin with a combination of AR extract and CTN could lead to potentiation of CDL permeation. CTN (1% w/v) enhanced the in vitro permeation of CDL across excised rat epidermis by 4.5-fold. The in vitro permeation of CDL in presence of mixture of AR extract and CTN was further enhanced to 6.9-fold (Table I).
Table II indicates that the in vitro permeation of CDL across epidermis excised from viable skin after 12 h treatment with any formulation was greater than that across epidermis excised at other time periods. However, comparison of this data with that presented in Table I indicates significantly less (p < 0.05) in vitro permeation of CDL across epidermis excised from viable skin treated with different formulations as compared to that across excised epidermis using the same formulations. This suggested that AR extract, CTN, or AR extract–CTN mixture was less effective in promoting the permeation of CDL when applied to viable skin. This could be attributed to the continuously weaning off of the effect after 12 h of application of any formulation as judged from the quantities of microconstituents returning to normal values in skin. The data summarized in Table III shows that all the microconstituents returned to their basal values after 12 h of application of AR extract, CTN, or AR extract–CTN mixture to viable skin. This observation is in consonance with earlier reports where the skin lipids were observed to return to their basal values in a bid to restore the permeability barrier status of the skin (31).
The normal (untreated) epidermis (Fig. 1 A) exhibited three endothermic transitions each at 70°C (T2), 80°C (T3), and 97°C (T4). The transition T1 (near 40°C) has been suggested to arise from minor structural rearrangements within the lipid bilayers of SC. However, reports suggest that endothermic transition T1 is not always detectable in all skin samples. The present investigation too revealed the absence of T1 endotherm in the thermogram of normal rat epidermis.
Treatment with PG/ETOH (7:3) mixture obliterated the T4 endotherm. T2 endotherm is reported to arise from lipids alone and represents changes in extended lamellar lipid domain of intercellular space (32). T3 endotherm is due to lipid–protein complex associated with corneocyte membrane (32,33). T4 endotherm is assigned to thermal denaturation of protein component located on surface of cell membrane or in intercellular spaces (34). The fourth endotherm T4 (near 100°C) has been assigned to thermal denaturation of a protein component located on the surface of cell membrane or in the intercellular spaces (35).
Treatment with PG/ETOH (7:3) mixture obliterated the T4 endotherm as evidenced in the DSC thermograms (Fig. 1 B). The T4 endotherm was also absent in thermograms of excised epidermis treated with aqueous solutions of AR extract (Fig. 1 C–G). This indicated that all the concentrations of AR extract affected the epidermal protein component similar to PG/ETOH mixture. The enthalpy of T2 was found to decrease after treatment with any concentration of AR extract (Table IV). The broad nature of endotherm in thermograms of AR extract-treated epidermis might be due to merger of T2 and T3 transitions (Fig. 1 C–G). Further, treatment with AR extract–CTN mixture obliterated all the endothermic transitions (Fig. 1 H), which revealed that the treatment resulted in almost complete extraction and fluidization of epidermal lipids. Hence, treatment with AR extract can be suggested to influence both epidermal lipids and proteins.
Table IV.
Thermotropic Attributes of T2 Endothermic Transition of Excised Rat Epidermis after Treatment with A. racemosus, Chitosan, or their Mixture
| Treatment | Endothermic transition (T 2) | Tm | |
|---|---|---|---|
| ΔH (Jg−1) | Decrease in ΔH (%) | ||
| 0.5% w/v AR | 21.59 ± 0.85 | 44.398 ± 0.79 | 68.02 ± 0.31 |
| 1% w/v AR | 21.98 ± 0.24 | 43.39 ± 0.32 | 68.12 ± 0.44 |
| 2.5% w/v AR | 21.9 ± 0.18 | 43.6 ± 0.25 | 70.51 ± 0.28 |
| 5% w/v AR | 21.03 ± 0.72 | 45.84 ± 0.86 | 64.14 ± 0.39 |
| 10% w/v AR | 9.14 ± 0.46 | 76.46 ± 0.51 | 66.26 ± 0.47 |
| AR (1% w/v)–CTN (1% w/v) mixture | No endotherm was observed | ||
| CTN (1% w/v) | 17.08 ± 0.28 | 56 ± 0.34 | 73.04 ± 0.43 |
A high TEWL indicates defects in the barrier function of the skin. As the skin barrier function is believed to be primarily located in the intercellular domains (1), the lipid phase acts as a barrier against water loss. Hence, it seems logical to correlate the increase in TEWL with the permeation of CDL across viable rat skin after treatments with AR extract.
The literature reveals importance of pore formation in epidermis during current assisted transdermal permeation of solutes (36). However, the density of pores has not been definitely elucidated through complementary morphological studies. The outermost layer of the skin, the SC, is believed to constitute the major barrier for drug permeation and is regarded as a heterogeneous two-compartment system composed of keratin-filled corneocytes embedded in an intercellular lipid matrix. This lipid matrix is organized in lamellar bilayers (37). These bilayers are formed by rearrangement and fusion of lamellar discs that are extruded into the intercellular regions from the uppermost cells of the stratum granulosum (38). The permeability barrier in the stratum corneum is provided by these lipid bilayers and corneocytes (39).
Different theoretical and experimental results have suggested that the drug penetration occurs through cavitation-induced keratinocytes or intercellular lipid bilayer disordering. SEM studies revealed loosening and creation of small pores in SC surface layers by AR extract, creation of larger pores by CTN, and both loosening and pore formation by AR extract–CTN mixture treatment in excised epidermis. The effect was of apparently less intensity when these formulations were applied to viable skin (Fig. 3). The TEM studies also revealed disordering of lipid areas (AR extract) and increase in intercellular space along with corneocyte detachment (AR extract–CTN mixture) in excised epidermis. These effects were apparently less severe when the treatments were given to viable skin (Fig. 4). It is important to note that these microscopic observations correlate very well with the findings of SC microconstituent estimations after similar treatments.
The results of pharmacodynamic study clearly indicate that the transdermal patches released the drug gradually over a period of time, which resulted in prolonged control of hypertension through 36 h. Oral administration of CDL rapidly reduced the blood pressure within 2 h. But the BP reduction stayed only for 8 h, after which the blood pressure rose to initial value steeply. The application of transdermal patches decreased the BP almost at the same rate as after oral administration and was able to maintain the reduced BP till 36 h (Fig. 5). Similarly, the plasma concentration of CDL was maintained above the Ceff level through 36 h after application of transdermal patch (Fig. 6). Hence, the prepared transdermal patch of CDL containing AR extract (1% w/v)–CTN (1% w/v) mixture were capable of surmounting the shortcomings of low bioavailability, short half-life, and high first-pass metabolism associated with its oral administration.
It has been reported that oral clinical doses of CDL produced Cmax of 23–79 ng/ml (40). However, Hokama et al. (41) reported Cmax of 297.7 ng/ml and 491.7 ng/ml after, respectively, 20 and 40 mg/kg oral dose of CDL to rats. The present investigation revealed attainment of Cmax of 121.04 ± 0.98 ng/mL after 10 mg/kg oral dose of CDL. The observed Cmax of 121.04 ± 0.98 ng/mL is approximately half of that reported by Hokama et al. This seems to be due to half the oral dose of CDL (10 mg/kg) used in the present investigation as against 20 mg/kg used by Hokama et al. (41). However, the Cmax attained after application of 40 mg dose of CDL in transdermal patches with AR extract (1% w/v) and CTN (1% w/v) as permeation enhancer was 430.06 ± 1.26 ng/mL. A higher dose of CDL in transdermal patches was employed keeping in view approximately 7-fold enhanced permeation of CDL using the enhancer system. Furthermore, the Cmax achieved after transdermal application of the patches (430.06 ng/ml) is quite close to that reported (490.7 ng/ml) by Hokama et al. (41) after oral administration of the same dose (40 mg/kg) of CDL. This suggests a significant improvement in system delivery of CDL through transdermal route using AR extract (1% w/v) and CTN (1%) w/v as enhancer system. Hence, the results of the present investigation suggest a promising role of AR extract in enhancing the percutaneous permeation of CDL due to its surfactant nature. CTN was found to potentiate the permeation-enhancing activity of AR extract. Further, the permeation-enhancing activity of AR extract and CTN could be suggested to be mediated through the combined effect on microconstituents of epidermis as well as alteration of epidermal ultrastructure as judged by SEM and TEM photographs. Hence, biochemical constitution and modification of epidermal ultrastructure seem to be inextricable aspects while understanding the percutaneous permeation activity of saponins containing extract of AR. Further, the normalization of contents of epidermal microconstituents after 48 h of application of AR extract, CTN, or AR extract–CTN mixture to viable skin rules out the possibility of irreversible damage by any of these agents.
CONCLUSION
The results of the present investigation revealed that transdermal patch of CDL containing AR extract (1% w/v) and CTN (1% w/v) was capable of enhancing the permeation of CDL across excised rat epidermis. Further, application of the patch to rats resulted in attainment of effective plasma CDL concentration, and the hypertension was controlled through 36 h. Biophysical (DSC), microscopic (SEM and TEM), biochemical, and TEWL investigations revealed the permeation-enhancing effect of AR extract–CTN mixture to be attributable to modification of lipid, proteins, and ultrastructure of the rat epidermis. Overall, the results suggested promising role of AR extract–CTN combination for use as an effective and safe percutaneous permeation enhancer in transdermal formulations.
Acknowledgement
We are thankful to Council of Scientific and Industrial Research (CSIR), New Delhi, [Scheme No. 01(2088)/06/EMR-II] for providing financial support. The authors thank AIIMS, New Delhi for providing their facility for conducting SEM and TEM investigations.
References
- 1.Barry B. W. Breaching the skin’s barrier to drugs. Nat. Biotechnol. 2004;22:165–167. doi: 10.1038/nbt0204-165. [DOI] [PubMed] [Google Scholar]
- 2.Pray W. S. Nonprescription Product Therapeutics. Philadelphia: Lippincott Williams & Wilkins; 2003. [Google Scholar]
- 3.Goodman M., Barry B. W. Action of penetration enhancers on human skin as assessed by the permeation of model drugs 5-fluorouracil and estradiol. I. Infinite dose technique. J. Invest. Dermatol. 1988;91:323–327. doi: 10.1111/1523-1747.ep12475655. [DOI] [PubMed] [Google Scholar]
- 4.Meier W., Hoppe U. Texture analysis of the surface of human skin. Skin Pharmacol. 1995;8:252–256. doi: 10.1159/000211355. [DOI] [PubMed] [Google Scholar]
- 5.Zatz J. L. Skin Permeation Fundamentals and Applications. Carol Stream, IL, USA: Allured Pub. Co.; 1993. [Google Scholar]
- 6.Shah V. P. Skin penetration enhancers: scientific perspective. In: Hsieh D. S., editor. Drug Permeation and Enhancement. New York, USA: Marcel Dekker; 1994. pp. 19–23. [Google Scholar]
- 7.Nokhodchi A., Shokri J., Dashbolaghi A., Hassan-Zadeh D., Ghafourian T., Barzegar-Jalali M. The enhancement effect of surfactants on the penetration of lorazepam through rat skin. Int. J. Pharm. 2003;250:359–369. doi: 10.1016/S0378-5173(02)00554-9. [DOI] [PubMed] [Google Scholar]
- 8.Babita K., Rana V., Tiwary A. K. Lipid synthesis inhibitors: effect on epidermal lipid conformational changes and percutaneous permeation of Levodopa. AAPS PharmSciTech. 2005;6:E473–E481. doi: 10.1208/pt060359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Babita K., Tiwary A. K. Transcutaneous delivery of levodopa: enhancement by fatty acid synthesis inhibition. Mol. Pharm. 2005;2:57–63. doi: 10.1021/mp049926u. [DOI] [PubMed] [Google Scholar]
- 10.Davar K. Drug-surfactant interactions: effect on transport properties. Int. J. Pharm. 1997;155:179–190. doi: 10.1016/S0378-5173(97)00162-2. [DOI] [Google Scholar]
- 11.Choi S., Jung S. Y., Kim C. H., Kim H. S., Rhim H., Kim S. C., Nah S. Y. Effect of Ginsenosides on voltage-dependent Ca2+ channel subtypes in bovine chromaffin cells. J. Ethnopharmacol. 2001;74:75–81. doi: 10.1016/S0378-8741(00)00353-6. [DOI] [PubMed] [Google Scholar]
- 12.Menin L., Panchichkina M., Keriel C., Olivares J., Braun U., Seppert E. K., Saks V. A. Macrocompartmentation of total creatine in cardiomyocytes revisited. Mol. Cell. Biochem. 2001;220:149–159. doi: 10.1023/A:1010960309898. [DOI] [PubMed] [Google Scholar]
- 13.Plock A., Sokolowska-Kohler W., Presber W. Application of flow cytometry and microscopical methods to characterize the effect of herbal drugs on Leishmania spp. Exp. Parasitol. 2001;97:141–153. doi: 10.1006/expr.2001.4598. [DOI] [PubMed] [Google Scholar]
- 14.Seeman P. Ultrastructure of membrane lesions in immune lysis, osmotic lysis and drug-induced lysis. Fed. Proc. 1974;33:2116–2124. [PubMed] [Google Scholar]
- 15.Seeman P., Cheng D., Iles G. H. Structure of membrane holes in osmotic and saponin hemolysis. J. Cell Biol. 1973;56:519–527. doi: 10.1083/jcb.56.2.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brain K., Hadgraft J., Al-Shatalebi M. Membrane modification in activity of plant molluscicides. Planta Med. 1990;56:663. doi: 10.1055/s-2006-961323. [DOI] [Google Scholar]
- 17.Schipper N. G. M., Olsson S., Hoogstraate J. A., deBoer A. G., Varum K. M., Artursson P. Chitosans as absorption enhancers for poorly absorbable drugs 2: mechanism of absorption enhancement. Pharm. Res. 1997;14:923–929. doi: 10.1023/A:1012160102740. [DOI] [PubMed] [Google Scholar]
- 18.Smith J., Wood E., Dornish M. Effect of chitosan on epithelial cell tight junctions. Pharm. Res. 2004;21:43–49. doi: 10.1023/B:PHAM.0000012150.60180.e3. [DOI] [PubMed] [Google Scholar]
- 19.Goodman M., Barry B. W. Action of penetration enhancers on human skin as assessed by the permeation of model drugs 5-fluorouracil and estradiol. I. Infinite dose technique. J. Invest. Dermatol. 1988;91:323–327. doi: 10.1111/1523-1747.ep12475655. [DOI] [PubMed] [Google Scholar]
- 20.Morgan T. Clinical pharamacokinetics and pharmacodynamics of carvedilol. Clin. Pharmacokinet. 1994;26:335–346. doi: 10.2165/00003088-199426050-00002. [DOI] [PubMed] [Google Scholar]
- 21.Kligman A. M., Christophers E. Preparation of isolated sheets of human stratum corneum. Arch. Dermatol. 1963;88:702–705. doi: 10.1001/archderm.1963.01590240026005. [DOI] [PubMed] [Google Scholar]
- 22.Folch J., Lees M., Sloane-Stanley G. H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957;226:497–502. [PubMed] [Google Scholar]
- 23.Sabbadini R., McNutt W. M., Jenkins G., Betto R., Salviati G. Sphingosine is endogenous to cardiac and skeletal muscle. Biochem. Biophys. Res. Commun. 1993;3:752–758. doi: 10.1006/bbrc.1993.1689. [DOI] [PubMed] [Google Scholar]
- 24.Singh S., Bi M., Jayaswal S. B., Singh J. Effect of current density on the iontophoretic permeability of benzyl alcohol and surface characteristics of human epidermis. Int. J. Pharm. 1998;166:157–166. doi: 10.1016/S0378-5173(98)00045-3. [DOI] [Google Scholar]
- 25.Van den Bergh B. A. I., Swartzendruber D. C., Geest A. B., Hoogstraate J. J., Schrijvers A. H. G. J., Bodde H. E., Junginger H. E., Bouwstra J. A. Development of an optimal protocol for the ultrastructural examination of skin by transmission electron microscopy. J. Microsc. 1997;187:125–133. doi: 10.1046/j.1365-2818.1997.2200779.x. [DOI] [PubMed] [Google Scholar]
- 26.Rogiers V. TEWL-measurements in patch test assessment: the need for standardisation. Current problems in dermatology. In: Elsner P., Maibach H. I., editors. Irritant Dermatitis, New Clinical and Experimental Aspects. Basel: Karger; 1995. pp. 152–158. [DOI] [PubMed] [Google Scholar]
- 27.Khazaei M., Nematbakhsh M. The effect of hypertension on serum nitric oxide and vascular endothelial growth factor concentrations. A study in DOCA-salt hypertensive overiectomized rats. Regulatory Pept. 2006;135:91–94. doi: 10.1016/j.regpep.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 28.Shokri J., Nokhodchi A., Dashbolaghi A., Hassan- Zadeh D., Ghafourian T., Jalali M. B. The effect of surfactants on the skin permeation of diazepam. Int. J. Pharm. 2001;228:99–107. doi: 10.1016/S0378-5173(01)00805-5. [DOI] [PubMed] [Google Scholar]
- 29.Gibaldi M., Feldman S. Mechanisms of surfactant effects on drug absorption. J. Pharm. Sci. 1970;59:579–589. doi: 10.1002/jps.2600590502. [DOI] [PubMed] [Google Scholar]
- 30.Nokhodchi A., Nazemiyeh H., Ghafourian T., Hassan-Zadeh D., Valizadeh H., Bahary L. A. S. The effect of glycyrrhizin on the release rate and skin penetration of diclofenac sodium from topical formulations. Il Farmaco. 2002;57:883–888. doi: 10.1016/S0014-827X(02)01298-3. [DOI] [PubMed] [Google Scholar]
- 31.Sapra B., Jain S., Tiwary A. K. Transdermal delivery of carvedilol containing glycyrrhizin and chitosan as permeation enhancers: biochemical, biophysical, microscopic and pharmacodynamic evaluation. Drug Deliv. 2008;15:443–454. doi: 10.1080/10717540802327047. [DOI] [PubMed] [Google Scholar]
- 32.Golden G. M., Guzek D. B., Harris R. R., McKie J. E., Potts R. O. Lipid thermotropic transitions in human stratum corneum. J. Invest. Dermatol. 1986;86:255–259. doi: 10.1111/1523-1747.ep12285373. [DOI] [PubMed] [Google Scholar]
- 33.Knutson K., Potts R. O., Guzek D. B., Golden G. M., Mckie J. E., Lambert W. J., Higuchi W. I. Macro and molecular physical chemical considerations in understanding drug transport in the stratum corneum. J. Control. Release. 1985;2:67–87. doi: 10.1016/0168-3659(85)90034-3. [DOI] [Google Scholar]
- 34.Van Duzee B. F. Thermal analysis of human stratum corneum. J. Invest. Dermatol. 1975;65:404–408. doi: 10.1111/1523-1747.ep12607656. [DOI] [PubMed] [Google Scholar]
- 35.Vaddi H. K., Ho P. C., Chan Y. W., Chan S. Y. Oxide terpenes as human skin penetration enhancers of haloperidol from ethanol and propylene glycol and their modes of action on stratum corneum. Biol. Pharm. Bull. 2003;26:220–228. doi: 10.1248/bpb.26.220. [DOI] [PubMed] [Google Scholar]
- 36.Yamashita N., Tachibana K., Ogawa K., Tsujita N., Tomita A. Scanning electron microscopic evaluation of the skin surface after ultrasound exposure. Anat. Rec. 1997;247:455–461. doi: 10.1002/(SICI)1097-0185(199704)247:4<455::AID-AR3>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 37.Bouwstra J. A., Cheng K., Gooris G. S., Weerheim A., Ponec M. The role of ceramides 1 and 2 in the stratum corneum lipid organization. Biochim. Biophys. Acta. 1996;1300:177–186. doi: 10.1016/0005-2760(96)00006-9. [DOI] [PubMed] [Google Scholar]
- 38.Lavker R. M. Membrane-coating granules: the fate of the discharged lamellae. J. Ultrastruct. Res. 1979;55:79–86. doi: 10.1016/S0022-5320(76)80083-4. [DOI] [PubMed] [Google Scholar]
- 39.Lampe M. A., Williams M. L., Elias P. M. Human epidermal lipid characterization and modulation during differentiation. J. Lipid Res. 1983;24:131–140. [PubMed] [Google Scholar]
- 40.Fujimaki M., Hakusui H., Hasegawa Y., Ajima H., Ota H., Igafashi S., Yamamura H. Pharmacokinetics of carvedilol (DQ-2466) in healthy subjects. Jpn. J. Clin. Pharmacol. Ther. 1990;21:415–424. [Google Scholar]
- 41.Hokama N., Hobra N., Kameya H., Ohshiro S., Sakanashi M. Rapid and simple micro-determination of carvedilol in rat plasma by high-performance liquid chromatography. J. Chromatogr. B. 1999;732:233–238. doi: 10.1016/S0378-4347(99)00248-0. [DOI] [PubMed] [Google Scholar]