Abstract
Background From 1948 to 1975, Norway had a mandatory tuberculosis (TB) screening programme with Pirquet testing, X-ray examinations and BCG vaccination. Electronic data registration in 1963–75 enabled the current study aimed at revealing (i) the relations between socioeconomic factors and tuberculosis infection and (ii) differences in later all-cause mortality according to TB infection status.
Methods TB screening data were linked to information from the Norwegian Cause of Death Registry (1975–98) and the National Population and Housing Censuses (1960, 1970 and 1980). Analyses were done for 10 years cohorts born 1910–49, separately for men (∼534 000 individuals) and women (608 000), using logistic and Cox regressions.
Results TB infection and X-ray data confirmed the strong regional pattern seen for TB mortality, with the highest rates in the three northernmost counties and higher rates in urban than rural areas. High socioeconomic status relates to lower odds both for TB infection and TB-related chest X-ray findings (odds ratios 0.6–0.7 for highest vs lowest educational groups). Those infected by TB, and especially those with chest X-ray findings, have increased all-cause mortality in at least a 20 years period following determination of tuberculin status (hazard ratios ∼1.15 and 1.30, respectively, higher for late than early cohorts).
Conclusions TB particularly affected lower socioeconomic strata, but even those in higher strata were at high risk. The differences in all-cause mortality could partly be attributed to socioeconomic factors, but we hypothesize that developing TB infection may also indicate biological frailness.
Keywords: Tuberculosis, mass screening, socioeconomic factors, mortality
Introduction
In the early 20th century, ∼20% of all deaths in Norway were caused by TB. Despite a continual and marked fall in incidence, the disease still caused 5% of all deaths in the first years following the Second World War.1 Motivated by the seriousness of the disease and new possibilities for case finding and treatment, a large scale mandatory screening and vaccination campaign was launched in 1948.2 In the later stages of this campaign, from 1963 to 1975, data was stored electronically and linked to a unique national identification number.3 The registration also included data from earlier screenings to assess TB infection, vaccination status, earlier active disease and TB-related chest X-ray findings.
The national identification number system enables linkage to other registries such as the Cause of Death Registry and the National population and housing censuses. Utilizing such linkage, our aims were (i) to reveal regional and socioeconomic differences in TB incidence for cohorts born 1910–49 (∼1.14 million individuals) and (ii) to examine later mortality differences between individuals with different TB infection status based on mortality information from the Cause of Death Registry for the years 1976–98.
Materials and methods
Tuberculosis control programme
In 1947, the Norwegian Parliament passed an act making participation in a national TB control programme compulsory for all >14 years of age. The programme was carried out by the National Mass Radiography Service using seven to eight mobile teams (buses and boats).3 After an initial all country campaign, counties were visited by intervals depending on the TB prevalence, ranging from 2 years (northernmost areas) to 10–12 (southern inland).
The control programme included the following elements:
Miniature chest X-ray. Films (35 mm/70 mm) were independently read by two specialists. The changes used to indicate TB-related findings were calcifications in lungs or hilus, pleural changes, pulmonary changes without calcifications or pleural changes, pulmonary infiltration and calcification, pulmonary infiltration and pleural changes.4 An average of ∼300 000 people were examined annually, corresponding to ∼80–85% of the eligible population. Approximately 5% had recently been examined by others (e.g. in military service), 5–10% were ill or temporarily absent, while ∼5% did not attend for unspecified reasons.3,5
Tuberculin test of all except those with health certificate confirming earlier positive reaction without previous vaccination. Von Pirquet's method was modified to make reactions more prominent by adding one drop of Adrenalin 1% per ml tuberculin.6 Five millimeter intradermal scratches were made through two drops of tuberculin applied on the volar surface of the left forearm. The skin reactions were noted by trained nurses after 48 h (maximum 72 h). Persons with one infiltration ≥4 mm were defined as tuberculin reactors.6
BCG-vaccination. Based on the prevailing opinion on the efficacy of BCG at that time, the programme included vaccination of all tuberculin non-reactors under 40 or 50 years.2 The vaccine underwent continuous quality control and was applied by intradermal injection.6 From 1948 to 1952 the annual number of vaccinations reached 120–150 000, then the number dropped to 80 000 as individuals previously vaccinated through the school health programme constituted a growing proportion (see also Supplementary Figure 1).
Measurement of height and weight to the nearest centimetre and half kilogram (not mandatory, from 1963).7
The last country-wide campaigns took place between 1963 and 1975. Data were registered electronically and linked to the 11-digit national identification number; 95% of these registrations were carried out in 1966–73. At attendance, results from previous examinations were added to the record, based on health certificates and in some cases verbal information (see also ‘Categories used’ below). These results included size of latest recorded tuberculin test, BCG-vaccination (including year of vaccination), TB-related chest X-ray findings, current or earlier active TB and exposure factors like year of birth, sex and marital status.
Linkage
The above data were linked to information from
Norwegian Cause of Death Registry8—information until 1998.
The national population and housing censuses9,10 from 1960, 1970 and 1980—information on education (own and spouse, 1960 and 1980), employment (own and spouse, 1960 and 1980), marital status and housing (county, type of municipality, type of neighbourhood, number in household, 1970).
The combined file includes information on ∼876 000 men and 954 000 women, out of which 534 000 and 608 000 were born between 1910 and 1949—the birth cohorts in focus. The data covers all of Norway, except Oslo (∼12% of the population, separate screening programme) and parts of two other counties (Buskerud and Bergen).
Statistical methods
Logistic regression was used to evaluate regional and socioeconomic differences in TB infection and X-ray findings. For survival analysis, we used both Poisson and Cox regression, however, results were similar and we only present results from Cox regression. The main analyses were carried out for ten year cohorts, separately for men and women, and with age as time variable in the Cox regressions (controlling for year of birth within the cohorts; regressions using calendar time gave similar results).
Categories used
For TB infection/vaccination status, the following main groups were identified:
BCG vaccinated. Information on vaccination or scar from vaccination (60% of the 1910–49 cohorts).
Non-confirmed BCG vaccination. Self-reported vaccination without confirmation (7%).
TB infected. Confirmed earlier positive test or adrenalin-Pirquet test result ≥4mm with no indication of BCG vaccination (24%).
Non-confirmed infection. Self-reported earlier TB infection (1%).
Uncertain. Lack of reliable information (8%).
In the logistic and Cox regression analyses on TB infection status, we only include the two groups with confirmed information: ‘BCG vaccinated’ (not naturally infected) and naturally ‘TB infected’. For TB-related chest X-ray findings, the control group consists of all without confirmed findings.
When comparing survival, we focus either on all cause mortality or on a subdivision into main disease groups denoted Cardiovascular disease (CVD; ICD9 390–459), Cancer (ICD9 140–239) and Other diseases. For the cohorts 1910–19 and 1920–29, we considered the years 1976–1998, while for the 1930–39 and 1940–49, we use 1981–98, due to incomplete information in the 1960 census for these cohorts combined with few death in 1976–80. Note that mortality was studied for a time period after all information on BCG and TB infection status had been collected.
The following exposure factors were used in the regression analyses (together with tuberculin status/TB-related chest X-ray findings):
County, area of residence (urban, rural) and type of municipality (central manufacturing/service, fishing, agricultural, mixed agricultural/manufacturing, other); these are collectively denoted geographical variables
Marital status (married, others).
Number in household.
Height (cm)
Body mass index (kg/m2) subdivided in groups BMI < 20, 20 ≤ BMI < 25, 25 ≤ BMI < 30, BMI ≥ 30.
Education grouped according to duration of education (number of scheduled years). Data from 1980 census if available, otherwise 1960 data.
Occupation. We used the main grouping of the Central Bureau of Statistics,9 merging groups that were similar with respect to the main outcome variables and had high exchange rates between the occupational groups from 1960 to 1980 (groups are given in Table 2). For men we used the 1960 census data for 1976–80 in the Cox analysis, then changing to 1980 data if available (thus employment was a time-dependent covariate). Since unemployment in 1980 might indicate ongoing serious disease, we excluded from the Cox regressions those unemployed in 1980 and where 1960 occupation could not be used as substitute (∼3%). For women from the cohorts 1910–19 and 1920–29 we used husbands occupation if available (few married with registered own occupation), otherwise own occupation. For later cohorts, we preferentially used own occupation (1980 or 1960 census), otherwise husband's occupation if available.
Table 2.
Odds ratios for TB infected vs not infected and TB-related X-ray findings vs no findings for different employment groups
| Infecteda |
X-ray findingsb |
Size of group (%) | |||
|---|---|---|---|---|---|
| Employment | 1920–29 | 1930–39 | 1920–29 | 1930–39 | First, second cohort |
| Manufacturing, transp, mining | 1.00 | 1.00 | 1.00 | 1.00 | 41, 44 |
| Technical, scientific, artistic | 0.80 | 0.77 | 0.97 | 0.83 | 9, 13 |
| Admin, sales, service | 0.93 | 0.90 | 1.10 | 0.91 | 19, 21 |
| Agriculture, forest, fishing | 0.75 | 0.77 | 0.86 | 1.00 | 11, 10 |
| Non-active, unknown | 1.14 | 1.12 | 1.22 | 1.23 | 19, 11 |
| Number used in regression | 122 024 | 100 484 | 147 809 | 119 885 | |
Results from logistic regression with employment, geographical variables and year of birth as covariates. Data for males, 1980 census.
aConfidence intervals sizes 0.03–0.06 on each side of the point estimates.
bConfidence interval sizes ∼0.10 for 1920–29, and 0.20 for 1930–39.
Results
TB infection and vaccination status
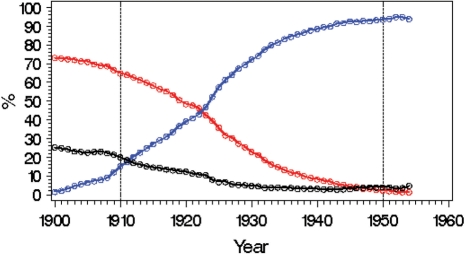
Figure 1 shows status for TB infection and BCG vaccination for different cohorts as revealed by the screenings from 1948 onwards. The curves represent a mix of a cohort effect and an age effect; however, since most TB infections occur below the age of 20–30 years, the cohort effects dominate. While the clear majority in cohorts from the first part of the century got infected, the fraction decreased rapidly from ∼1910, reaching levels of ∼5% in the first cohorts following World War II. The population screening programme was replaced by selective screening in the mid-1970s due to few cases of new TB infections acquired in Norway.11
Figure 1.
TB infection for birth cohorts in Norway. Red curve: percentage of TB infected (confirmed or self-reported); Blue curve: percentage BCG vaccinated (confirmed or self reported); Black curve: percentage uncertain
Regional differences
The frequency of TB related chest X-ray findings decreased rapidly and roughly linearly from ∼6% for those born ∼1910 to ∼1.5% for the cohort of 1930, and then fell gradually to ∼0.4% for the first post-war cohorts (Supplementary Figure 2).
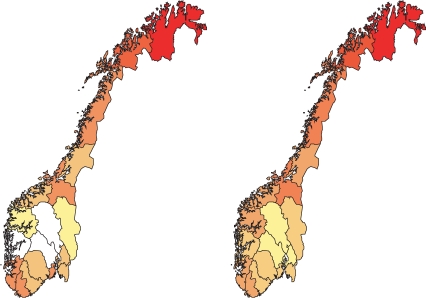
Figure 2 shows the regional distribution of TB infection and TB mortality. The distributions of TB infection are for the 1910–19 cohort, while mortality relates to 1931–35 (when average cohort age approached 20 years). The TB infection and mortality data reveal the same over-all pattern with the most serious epidemic in the north, while the southern inland have lower levels of TB infection and mortality. The same broad picture is seen for TB-related chest X-ray findings, although the distribution is more uncertain. This regional pattern is representative for cohorts born after ∼1910. Earlier the highest mortalities were found in south-western areas, while mortality has remained low in the southern inland from the earliest reliable registrations ∼1880 (Supplementary Figure 3). Incidence was lower in rural compared with urban areas, as revealed by low odds ratios for rural vs urban areas of residence [e.g. 0.68 (0.66–0.70) for men and 0.75 (0.73–0.78) for women from 1920 to 1929 cohort, Supplementary Table 1].
Figure 2.
Left panel: Distribution of TB infected individuals, male cohort born 1910–19 (yellow: <58% infected, dark red: ≥80% infected). Right panel: TB mortality rates, male cohort born 1931–35, (yellow: ≤10 per 10 000 inhabitants, dark red: >16 per 10 000 inhabitants). White: unreliable information
The regional patterns of TB infection and mortality show similarities to the pattern later seen for the lifestyle related ‘epidemic’ of CVD. Thus, the northern regions also had highest CVD mortality, and among the nine counties in the upper half with respect to TB infection rates, seven were also in the upper half with respect to CVD mortality (1920–29 cohort, males, see Supplementary Figure 4).
Socioeconomic differences
Table 1 shows differences between educational groups in the odds of being affected by TB for the cohorts born 1920–29 and 1930–39; results for other cohorts are similar.
Table 1.
Odds ratios for TB infected vs not infected (upper panel) and TB-related chest X-ray findings vs no findings (lower panel) for different educational levels
| 1920–29 |
1930–39 |
||||
|---|---|---|---|---|---|
| Educational level | Men | Women | Men | Women | Size of groups (%) |
| Infected vs vaccinateda | |||||
| Compulsory (max. 9 years) | 1.00 | 1.00 | 1.00 | 1.00 | 50, 60, 43, 49 |
| 10 years | 0.89 | 0.85 | 0.86 | 0.76 | 22, 29, 23, 37 |
| 11–12 years | 0.89 | 0.80 | 0.83 | 0.78 | 18, 5, 21, 5 |
| 13–16 years | 0.82 | 0.77 | 0.74 | 0.63 | 7, 5, 9, 8 |
| Master level (17+) | 0.62 | (0.62) | 0.61 | (0.61) | 3, 0.3, 4, 0.4 |
| Population size in regression | 122 024 | 134 147 | 100 484 | 113 040 | |
| With X-ray findings vs no findingsb | |||||
| Compulsory (max. 9 years) | 1.00 | 1.00 | 1.00 | 1.00 | 50, 59, 42, 48 |
| 10 years | 1.06 | 0.80 | 1.05 | 0.76 | 22, 29, 23, 37 |
| 11–12 years | 0.96 | 0.69 | 0.98 | (0.73) | 18, 6, 22, 6 |
| 13–16 years | 0.87 | 0.61 | 0.75 | 0.62 | 7, 5, 10, 8 |
| Master level (17+) | 0.67 | – | (0.77) | (0.63) | 3, 0.3, 4, 0.5 |
| Population size in regression | 147 809 | 163 874 | 119 885 | 136 687 | |
Results are from logistic regression with educational level, geographical variables and year of birth as covariates. ‘Size of group’ gives percentage in each educational group for each of the four cohort/gender groups.
aCIs 0.03–0.06 on each side (except uncertain ratios in parentheses).
bCIs 0.07–.18 on each side (except uncertain ratios in parentheses).
Those with high education have markedly lower odds both for TB infection and TB-related chest X-ray findings. Note, however, that the high education groups are fairly small (Table 1, last columns). Somewhat larger group differences are indicated for women than men, but this may partially be attributed to stronger selection among women as indicated by the lower fraction of women in the higher educational groups.
A similar picture appears when considering occupational groups (Table 2). Those in technical and scientific ‘high status’ occupations have low odds, while the highest odds are found among unemployed. Manual workers also have high odds, while employees in sales and service are at intermediate levels. Those employed in ‘agriculture and fishing’, a group where TB infection pressure is likely to be low, had low odds. The effect of social conditions and lifestyle is also indicated by married individuals having lower odds for TB infection than the unmarried [e.g. OR 0.86 (0.81–0.91) for males in cohort 1930–39]. Furthermore, increasing height is associated with lower odds [OR 0.94 (0.92–0.97) for 10 cm increase, Supplementary Table 2].
Thus, TB clearly affected the lower socio-economic strata most severely, however, given the strong over-all impact of TB, the figures also illustrates that the disease was a serious threat to all parts of society. Note also that if regional variables are neglected in the analysis, differences between socioeconomic groups decrease since those with higher educations tend to live in densely populated areas with high TB infection rates.
All-cause mortality
Table 3 shows hazard ratios from Cox regression for a period of ∼20 years following determination of tuberculin status. The hazards of infected individuals are above 1.0 for both sexes and all cohorts. These ratios are obtained with all available exposure variables included; this inclusion reduced excess mortality by ∼20–30% compared with models with only year of birth and county (see Supplementary Table 3).
Table 3.
Hazard ratios for mortality from Cox regression for infected individuals compared with vaccinated (not infected) individuals
| Cohort | Men | Women |
|---|---|---|
| All-cause mortality | ||
| 1910–19 | 1.07 (1.05–1.09) | 1.09 (1.07–1.11) |
| 1920–29 | 1.11 (1.09–1.13) | 1.12 (1.08–1.15) |
| 1930–39 | 1.15 (1.10–1.21) | 1.09 (1.03–1.16) |
| 1940–49 | 1.13 (1.02–1.26) | 1.18 (1.02–1.37) |
| CVD mortality | ||
| 1910–19 | 1.07 (1.04–1.11) | 1.10 (1.06–1.14) |
| 1920–29 | 1.12 (1.08–1.16) | 1.15 (1.08–1.22) |
| 1930–39 | 1.11 (1.02–1.21) | – |
| Cancer mortality | ||
| 1910–19 | 1.07 (1.03–1.11) | 1.04 (0.99–1.09) |
| 1920–29 | 1.14 (1.09–1.19) | 1.06 (1.01–1.11) |
| 1930–39 | 1.19 (1.09–1.31) | – |
| Other causes | ||
| 1910–19 | 1.07 (1.04–1.10) | 1.11 (1.07–1.15) |
| 1920–29 | 1.08 (1.05–1.12) | 1.15 (1.10–1.29) |
| 1930–39 | 1.15 (1.08–1.24) | – |
Table 4 shows hazard ratios for mortality associated with TB-related chest X-ray findings. All are above 1.0, and the ratios for X-ray findings are markedly higher than for those only infected. Ratios tend to be higher for late cohorts, likely related to the smaller fractions of TB infected/X-ray positive in these cohorts. The cause specific mortality estimates are uncertain, but as expected show high ratios for lung related diseases, like emphysemas, bronchitis and lung cancer. However, high ratios are also found for broad disease groups, e.g. the higher ratios for woman than men (Table 4) are to a large extent caused by CVD. The ratios in Table 4 are obtained with all exposure variables in the model; excess mortality for those with TB-related chest X-ray findings is moderately higher than the table values if only year of birth and county are included (Supplementary Table 3).
Table 4.
Hazard ratios for all cause mortality from Cox regression for individuals being infected but without X-ray findings and for individuals with TB related X-ray findings; vaccinated individuals constitute the reference group
| Infected, but no X-ray findings | With TB related X-ray findings | |
|---|---|---|
| Men | ||
| 1910–19 | 1.07 (1.05–1.09) | 1.18 (1.14–1.23) |
| 1920–29 | 1.10 (1.08–1.13) | 1.28 (1.20–1.36) |
| 1930–39 | 1.15 (1.10–1.21) | 1.29 (1.09–1.52) |
| Women | ||
| 1910–19 | 1.09 (1.06–1.11) | 1.34 (1.28–1.40) |
| 1920–29 | 1.11 (1.08–1.14) | 1.41 (1.30–1.53) |
| 1930–39 | 1.08 (1.02–1.15) | 1.90 (1.57–2.31) |
Discussion
Our data on TB infection confirm the marked regional pattern reported for TB mortality, with especially high rates in the three northernmost counties and higher rates in urban than rural areas. Moreover, high socioeconomic status relates to lower odds both for TB infection and TB-related chest X-rays findings. Analysis of all-cause mortality in a 20 years period following determination of tuberculin status reveals that individuals infected by TB and especially those with positive TB-related chest X-ray findings have increased death rates.
Regional and socioeconomic differences in TB infection rates
From ∼1880 when mortality statistics are reliable,1 TB mortality rates has shown a clear regional pattern. For later periods, the pattern is confirmed by morbidity data (according to The Tuberculosis Act of 1900, all new cases of TB treated by a doctor should be notified) and the current TB infections and X-ray data. In the last part of the 19th century, the south-western regions were most affected, probably related to a high level of trade-related contacts to high incidence areas like UK. Later, the highest rates were found in the north; probably partly reflecting the less favourable living and health care conditions in these areas. The general effects of the socioeconomic conditions are illustrated by Tables 1 and 2, showing marked differences between individuals with different educational or occupational background. These observations fit well to observations by Scheel and Heimbeck12,13 covering somewhat earlier cohorts. Their pioneering studies compared samples from Oslo (urban) and Hedmark (rural) in the mid-1920s. While nearly all above 40 years of age were infected in Oslo (according to Pirquet testing), some 20% remained uninfected in Hedmark, and the age with 50% infected differed by ∼7 years (18 and 25, respectively). Moreover, by comparing individuals from Oslo with jobs indicating high and low socioeconomic status, Scheel and Heimbeck found a 8 years difference in median age at TB infection. More recent studies from the western world have also demonstrated a relation between TB and socioeconomic conditions, as revealed for instance by a large case–control study from USA covering the years 1987–93.14 Studies from developing countries also reveal socioeconomic gradients, although the results may be less clear, probably due to other factors dominating over western measures of socioeconomic status.15,16
TB infection rates dropped markedly throughout the studied period and continued to drop for cohorts born after 1950. In absolute terms, most of the fall was prior to the screening and vaccination campaigns after World War II, and was probably mainly caused by better isolation of cases,17 improved hygiene and nutrition. However, the relative reduction in mortality and new TB cases accelerated when screenings started after World War II,11 and assessments indicate a clear protective effect of vaccination.2,18 These latter studies, however, only evaluated the effect of vaccination at young ages, and the effect of vaccination at older ages remains uncertain.19
Mortality differences
Marked differences in all-cause mortality are seen between vaccinated, TB infected, and those with TB-related chest X-ray findings. The differences are found for both sexes and all cohorts. If comparing individuals with or without several life-threatening diseases in Norway, one will thus tend to find that being infected by TB appears as a ‘risk factor’ and BCG vaccination as ‘protective’.
Since TB infection is related to socioeconomic conditions and socioeconomic conditions strongly influence mortality, part of the mortality difference is undoubtedly related to socioeconomic differences not fully accounted for by the variables entered in our models. Most significantly, we lack information on smoking, known for broad and serious effect on mortality and with high rates of use especially among men (1920–29 cohort in Norway: for men >70% at ages 20–40 declining toward 40% at age 60; for women similar rates were 25–40%20). Concerning the mortality difference between those TB infected and not infected, such environmental factors (if optimally monitored) may explain a large part, even all, of the difference.
For TB-related chest X-ray findings, entering the available exposure variables in the regression models had fairly small effects. However, the fact that smoking may promote the development of TB21,22 may complicate the interpretation of the results. Still, considering only diseases known to be moderately affected by socioeconomic factors and smoking, such as many cancers, there were clear mortality differences. Thus, developing infection by mycobacteria may indicate some biomedical frailness, genetic or acquired, implying that infected individuals for given environmental conditions may have increased mortality from a class of diseases. It appears unlikely that mild and transient TB with or without lung scarring in itself has long-term negative effects on general mortality. Although some effects of the chest X-ray doses given during screening and follow-up controls cannot be ruled out, large-scale effects on mortality seem unlikely. Based on observations of all-cause mortality in children in developing countries it has been suggested that early vaccination is beneficial.23,24 As far as we know there is no evidence for long-term non-specific effects of vaccination of adults (in this case up to 40–50 years at vaccination). We rather suspect that those with signs of previous TB infection—especially presence of TB-related chest X-ray findings—are prone to some general constitutional weakness (for example sub-optimal immune protection) that makes them frail to several disease groups.
Methodological considerations and limitations
The screening programme covered most of the country, however not the largest urban area (Oslo with ∼12% of the Norwegian population). Total coverage for each screening round is given as 80–85% of the population above 15 years in the screened areas, but somewhat varying over time, age groups, sex and areas.2,5 While the first and the last screening campaigns covered all of the country (except Oslo), intermediate screenings focussed on high incidence areas.3 For such reasons, the fraction of missing information especially on tuberculin status is unequally distributed. However, omitting areas with high fraction of missing information had minor effect on results, as had exclusion of areas with especially high TB infection rates. We also note that ∼16% with uncertain information were excluded in the main analysis on TB infection. We also made regression analyses including these groups; they tended to behave like the TB-infected group, although with some variation between the subgroups.
The size of the Pirquet reaction was only available for the most recent recording. Thus, the size was measured at highly varying times relative to BCG vaccination or TB infection; in the case of infections the time span is normally long and unknown. This is probably an important reason why reaction size showed no consistent relation to other outcomes, including outcomes directly related to TB such as chest X-ray findings. We therefore considered the lack of relation to outcome variables as a data shortcoming, rather than a medically relevant finding.
Conclusions
With the high impact of TB in the early part of the 20th century in Norway—one out of five deaths caused by TB and 60% of these deaths occurring before the age of 30—TB was a particularly serious threat in lower social strata, but even those in higher strata were strongly affected.
Marked differences in all-cause mortality were seen between vaccinated, TB infected, and those with TB-related chest X-ray findings. Part of these mortality differences are related to socioeconomic conditions. However, the strong relation between chest X-ray findings and later all-cause mortality may indicate that developing infection by mycobacteria may indicate some biomedical frailness, genetic or acquired, implying that infected individuals for given environmental conditions may have increased mortality from several disease groups.
Supplementary Data
Supplementary data are available at IJE online.
Acknowledgement
We would like to thank Kjell Bjartveit and Marthe Mæhlen for very valuable comments on the manuscript, and Espen Jettestuen for help with preparation of the data.
Conflict of interest: None declared.
KEY MESSAGES.
From 1948 to 1975, Norway had a mandatory TB screening programme including Pirquet testing, X-ray examinations and BCG vaccination. Data registrations were based on a national identification number enabling linkage to other registries.
High education and high status jobs were linked to markedly reduced TB infection rates. Still, being prevalent in young people and causing 20% of all death in the early 20th century, TB was a very serious threat to all parts of society.
In a 20 years period following determination of tuberculin status, those infected by TB and especially those with TB related chest X-ray findings had markedly increased all-cause mortality.
Supplementary Material
References
- 1.Backer JE. Dødeligheten og dens årsaker i Norge 1856–1955. Samfunnsøkonomiske studier 10, Central Bureau of Statistics, Oslo 1963 (in Norwegian) [Google Scholar]
- 2.Bjartveit K, Waaler HT. Some evidence of the efficacy of mass BCG vaccination. Bull WHO. 1965;33:289–319. [PMC free article] [PubMed] [Google Scholar]
- 3.Bjartveit K. Mass miniature radiography in Norway, today and in the future. Scand J Resp Dis. 1972;80(Suppl):31–42. [PubMed] [Google Scholar]
- 4.Bjartveit K, Foss OP, Gjervig T. The cardiovascular disease study in Norwegian counties. Results from first screening. Acta Med Scand. 1983;675(Suppl):1–184. [PubMed] [Google Scholar]
- 5.Statens skjermbildefotografering. Årsberetning for 1951. [Annual report 1951]. Oslo, 1954 (in Norwegian) [Google Scholar]
- 6.Waaler HT, Galtung O, Mordal K. The risk of tuberculosis infection in Norway. Tuberculosis surveillance Research Unit. Report no. 3. Bull Int Un Tuberc. 1975;1:5–61. [PubMed] [Google Scholar]
- 7.Bjartveit K, Foss OP, Gjervig T, Lund-Larsen PG. The cardiovascular disease study in Norwegian counties. Background and organization. Acta Med Scand. 1979;634(Suppl):1–70. [PubMed] [Google Scholar]
- 8.Gjertsen F. Dødsårsaksregisteret—en viktig datakilde for medisinsk forskning. Tidskr Nor Laegeforen. 2002;122:2551–54. (in Norwegian) [PubMed] [Google Scholar]
- 9.Central Bureau of Statistics. Population and Housing Census 1980, Vol II: Employment Statistics. Oslo: 1982. [Google Scholar]
- 10.Central Bureau of Statistics. Population and Housing Census 1980, Vol IV: Main results of the Censuses 1960, 1970 and 1980. Oslo: 1986. [Google Scholar]
- 11.Bjartveit K. The tuberculosis situation in Norway. Scand J Resp Dis. 1978;102(Suppl):28–35. [PubMed] [Google Scholar]
- 12.Heimbeck J. Tuberkuloseinfektion og tuberkulosevakcination. Tidsskr Nor Laegeforen. 1928;48:946–61. (in Norwegian) [Google Scholar]
- 13.Bjartveit K. Olav Scheel and Johannes Heimbeck: their contribution to understanding the pathogenesis and prevention of tuberculosis. Int J Tuberc Lung Dis. 2003;7:306–11. [PubMed] [Google Scholar]
- 14.Cantwell MF, McKenna MT, McCray E, Onorato IM. Tuberculosis and race/ethnicity in the United States; impact of socioeconomic status. Am J Respir Crit Care Med. 1997;157:1016–20. doi: 10.1164/ajrccm.157.4.9704036. [DOI] [PubMed] [Google Scholar]
- 15.Bates I, Fenton C, Gruber J, et al. Vulnerability to malaria, tuberculosis and HIV/AIDS infection and disease. Part 1: determinants operation at individual and household level. Lancet Infect Dis. 2004;4:267–77. doi: 10.1016/S1473-3099(04)01002-3. [DOI] [PubMed] [Google Scholar]
- 16.Lienhardt C, Fielding K, Sillah JS, et al. Investigation of the risk factors for tuberculosis: a case–control study in three countries in West Africa. Int J Epidemiol. 2005;34:914–23. doi: 10.1093/ije/dyi100. [DOI] [PubMed] [Google Scholar]
- 17.Wilson LG. Commentary: medicine, population, and tuberculosis. Int J Epidemiol. 2005;34:521–24. doi: 10.1093/ije/dyh196. [DOI] [PubMed] [Google Scholar]
- 18.Tverdal A, Funnemark E. Protective effect of BCG vaccination in Norway 1956-73. Tubercle. 1988;69:119–23. doi: 10.1016/0041-3879(88)90074-8. [DOI] [PubMed] [Google Scholar]
- 19.Joint Tuberculosis Committee of the British Thoracic Society. Control and prevention of tuberculosis in the United Kingdom: Code of practice 2000. Torax. 2000;55:887–901. doi: 10.1136/thorax.55.11.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rønneberg A, Lund KE, Hafstad A. Lifetime smoking habits among Norwegian men and women born between 1890 and 1974. Int J Epidemiol. 1994;23:267–76. doi: 10.1093/ije/23.2.267. [DOI] [PubMed] [Google Scholar]
- 21.Davies PDO, Yew WW, Ganguly D, et al. Smoking and tuberculosis: the epidemiological association and immunopathogenesis. Trans R Soc of Trop Med Hyg. 2006;100:291–98. doi: 10.1016/j.trstmh.2005.06.034. [DOI] [PubMed] [Google Scholar]
- 22.Bates MN, Khalakdina A, Pai M, et al. Risk of tuberculosis from exposure to tobacco smoke. A systematic review and meta-analysis. Arch Intern Med. 2007;167:335–42. doi: 10.1001/archinte.167.4.335. [DOI] [PubMed] [Google Scholar]
- 23.Aaby P, Samb B, Simondon F, et al. Non-specific beneficial effects of measles immunisation: analysis of mortality studies from developing countries. Br Med J. 1995;311:481–85. doi: 10.1136/bmj.311.7003.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roth AE, Stenballe LG, Garly ML, Aaby P. Beneficial non-targeted effects of BCG—ethical implications for the coming introduction of new TB vaccines. Tuberculosis. 2006;86:397–403. doi: 10.1016/j.tube.2006.02.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.