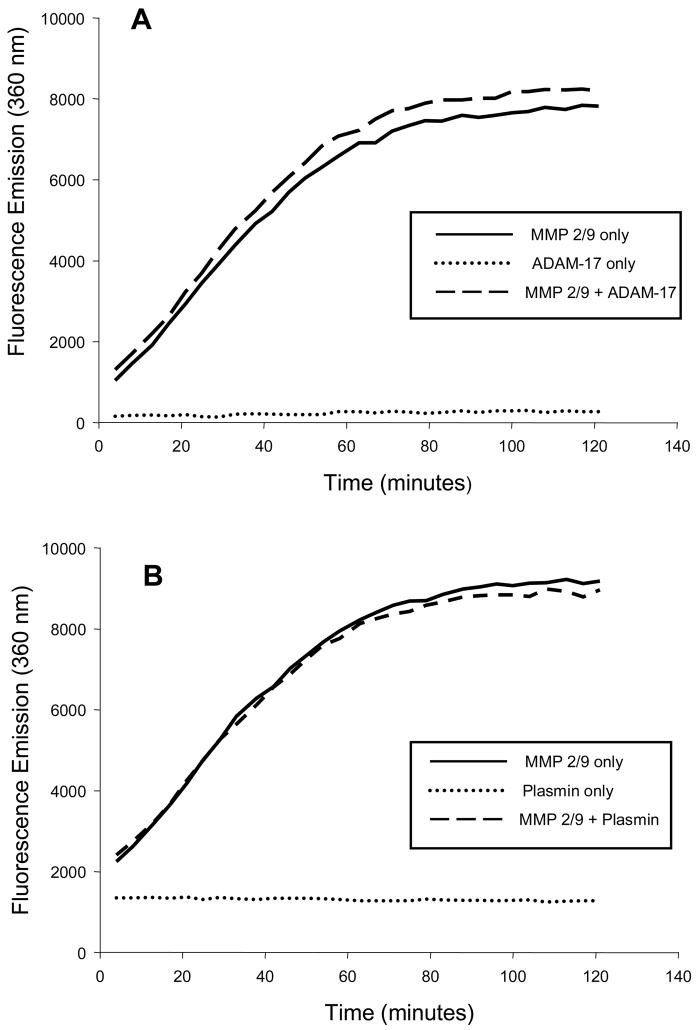
Figure 2.
In order to further examine the specificity of the MMP substrate (0.03 mM) was incubated in the presence of (A) a disintegrin and metalloprotease, ADAM-17 (1.25 ug/mL, R&D Systems, #930-ADB), or (B) with the serine protease plasmin (1.25 ug/mL, Sigma, #P1867) and fluorescence emission continuously monitored at 37degC. There was no fluorescence emission detected with either of these non-MMP proteases (dotted line). However, with co-incubation of the protease and the MMP 2/9 catalytic domain (1.25 ug/mL), fluorescence emission consistent with cleavage of the MMP substrate was achieved (dashed line).