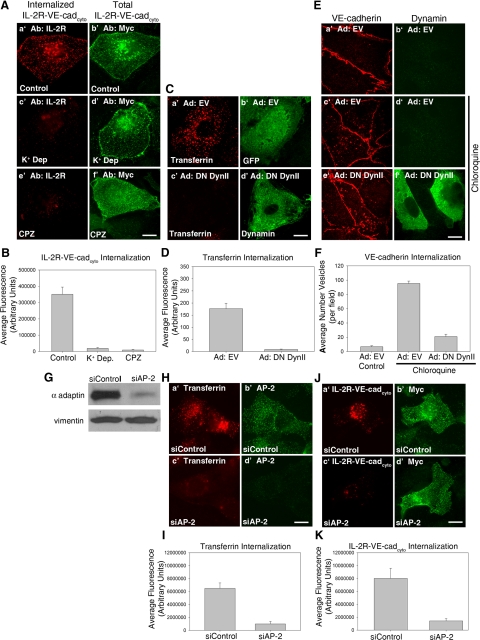
Figure 1.
VE-cadherin endocytosis is mediated through a clathrin-, AP-2–, and dynamin-dependent pathway. (A and B) Cell surface IL-2R-VE-cadcyto was labeled with IL-2R antibodies at 4°C. Cells were incubated at 37°C for 5 min, washed in a low pH buffer at 4°C to remove surface bound antibody, and then processed for immunofluorescence to visualize intracellular IL-2R-VE-cadcyto (A, a′, c′, and e′). Antibodies against the myc epitope tag at the carboxyl terminal domain of the IL-2R-VE-cadcyto were used to verify expression of the polypeptide (A, b′, d′, and f′). Note that untreated cells exhibited high levels of internalized IL-2R-VE-cadcyto, whereas treatment with K+ depletion or chlorpromazine completely inhibited internalization. (C–F) MECs were infected with adenoviruses expressing either GFP (C, a′ and b′) or dominant negative DynII (C, c′ and d′). The DynII completely blocked internalization of Alexa Fluor 555–conjugated transferrin (C and D). To monitor internalization of VE-cadherin, MECs were either untreated (E, a′ and b′) or treated with 100 μM chloroquine for 3 h (E, c′–f′). Note that intracellular vesicular accumulation of VE-cadherin is reduced in cells expressing DynII (E, e′ and f′, and F). (G–K) HMEC-1 cells were transfected with an siRNA oligo targeted to the AP-2 μ subunit or a nontargeting control siRNA. Lysates prepared from cells treated with control siRNA or AP-2 siRNA were separated by SDS-PAGE and immunoblotted for AP-2 to confirm AP-2 knockdown (G). Western blot and immunofluorescence reveals ∼75% reduction in AP-2 levels in siRNA-treated cells compared with control, as well as an 85% decrease in transferrin internalization (G–I). IL-2R-VE-cadcyto internalization assays were performed and AP-2 siRNA-treated cells (J, c′ and d′) exhibited an 80% decrease in internalization of IL-2R-VE-cadcyto compared with control (J, a′ and b′), indicating a requirement for AP-2 in VE-cadherin internalization (K). Error bars, SEM; n > 20 cells. Scale bar, 20 μm.