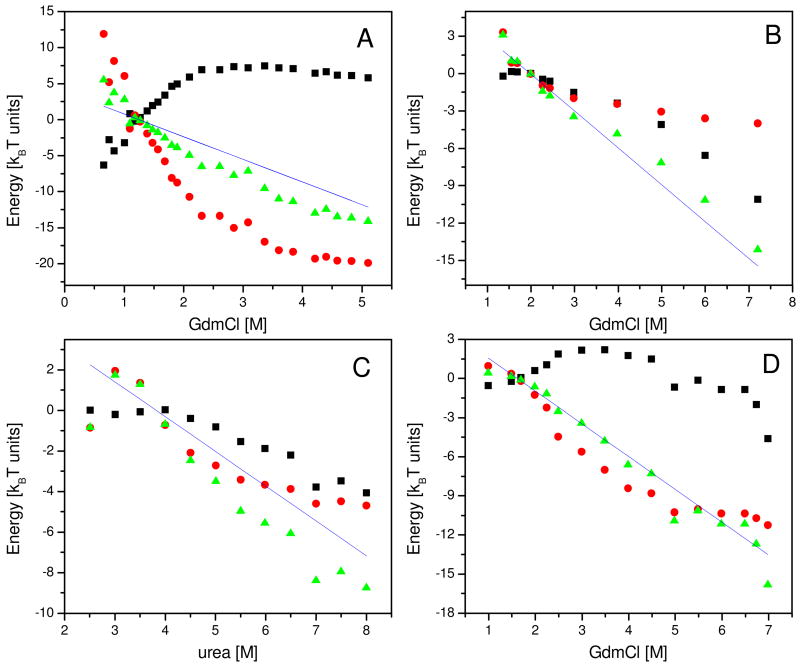
Figure 2.
Thermodynamic functions of collapse calculated from smFRET results for four different proteins. Values of the free energy of collapse, − ΔGU →C, as a function of denaturant concentration, are shown as green triangles. The enthalpic contributions −ΔEU →C and the entropic contributions kBT · ΔSU →C are shown as black squares and red circles, respectively. The interpolated value of each thermodynamic function at D50 is subtracted from all points. Solid lines show the denaturant dependence of the free energy of folding, ΔGU →N, as published in the literature. A. Results for protein L denatured in GdmCl (labeled 4a in Table 1). B. Im9 denatured in urea (3 in Table 1). C. CspTm denatured in GdmCl (2a in Table 1). D. Barstar denatured in GdmCl (1a in Table 1).