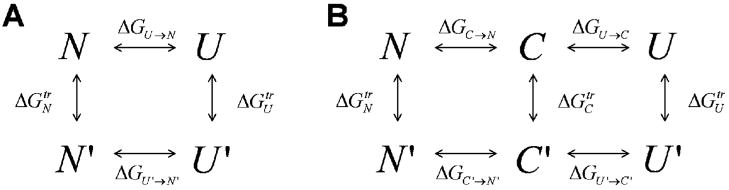
Scheme 1.
Thermodynamic cycles for unfolding. A. The well-known thermodynamic cycle relating two states, native (N) and unfolded (U), at two denaturant concentrations D (top) and D′ (bottom). We use the notation U and U′ for the unfolded state at the two concentrations, to stress that in fact a different ensemble of conformations is sampled in each. Thus, the transfer energy is composed of the enthalpy of transfer and the change in the conformational entropy of the unfolded state ensemble. B. For the calculation of the free energy of collapse, we introduce a new reference state, which is designated the maximally compact denatured state (C). Notice that in general this state does not equal the unfolded state at zero D.