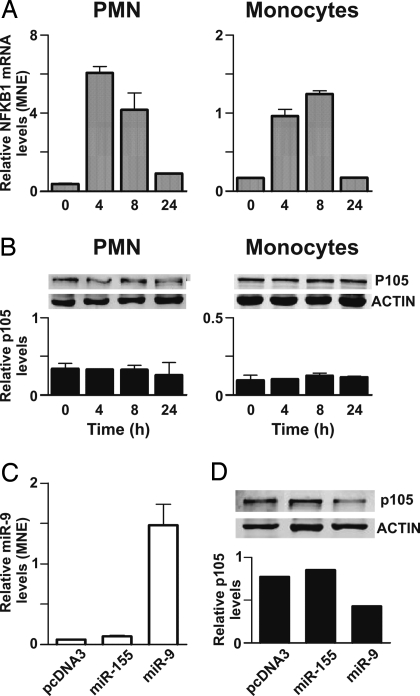
Fig. 6.
Analysis of the endogenous miR-9 target: NFKB1 mRNA and protein expression. (A) PMN and monocytes were cultured in the presence of LPS for the indicated times. Total RNA was purified and used to assay NFKB1 mRNA expression by RT-qPCR, as described in Materials and Methods. Relative NFKB1 gene expression is depicted as MNE units after GAPDH normalization. Data reported are representative of 3 independent experiments. (B) Whole-cell extracts (20 μg) were usually loaded on gels and immunoblots were performed by simultaneously using Abs specific for NFKB1 and Abs specific for actin, followed by incubation with Alexa Fluor-680 goat anti-rabbit Abs. One experiment representative of 3 is shown. The relative NFKB1/p105 levels (± SD, n = 3), quantified as described in SI Materials and Methods, are reported below each panel. (C) 6 × 106 monocytes were transfected with 5 μg of pcDNA3 empty vector, miR-155-encoding vector, or miR-9-encoding vector as described in Material and Methods. 48 hours posttransfection total RNA was purified and analyzed for miR-9 expression by RT-qPCR. Relative miR-9 expression is represented as MNE units after let-7a normalization. (D) Fifteen micrograms of whole cell extracts, purified from transfected monocytes cultured in the same conditions as in (C), were usually loaded on gels and NFKB1/p105 protein was detected as described above. The relative NFKB1/p105 levels, normalized for the total actin, are reported below the Western blot. (C and D) One experiment representative of 2.