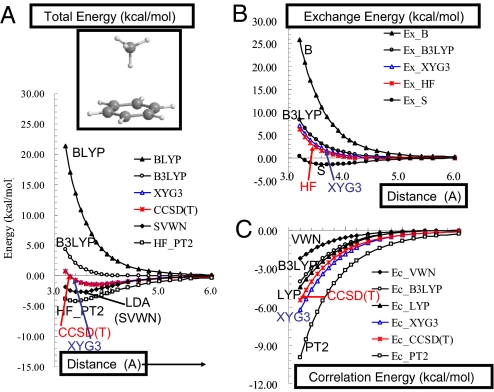
Fig. 2.
Enlarged figure for CH4-C6H6 system (see Fig. S1). (A) The intermolecular potentials for the CH4–C6H6 complexes from various methods. The most accurate is expected to be CCSD(T), which is at the complete basis set limit from ref. 36. XYG3 nearly superimposes on CCSD(T). B3LYP calculations were performed self-consistently by using the 6-311+G(3df,2p) basis set. All other results used density and orbitals from B3LYP. (B) The exchange part of the interaction energy. The HF result is expected to be the most accurate. The exchange part of XYG3 nearly superimposes on the HF. (C) The correlation part of the interaction energy. The CCSD(T) results are expected to be the most accurate. XYG3 is closest among the DFT.