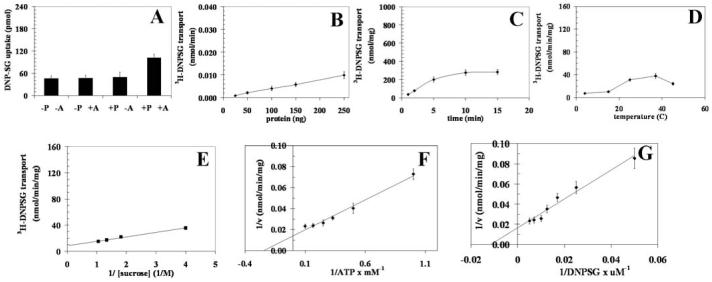
Figure 2.
Transport of 3H-DNPSG by RLIP76 purified from K562 cells. Purified RLIP76 was reconstituted into artificial liposomes and uptake of radiolabeled 3H-DNP-SG by control- or RLIP76-proteoliposomes was measured in the presence or absence of ATP. Vesicles equivalent to 250 ng of purified RLIP76 were used per 30 μl filtered reaction mixture in all panels except B. Total radioactivity retained by 0.45 μm filters in 96-well plates was determined after solubilizing filters in scintillation fluid, converted to pmol using the specific activity of 3H-DNPSG (3.6×103 cpm/nmol). Each point represents an average and SD calculated from 12 measurements [control (-P) or RLIP76-proteoliposomes (+P), without (-A) or with ATP (+A), in triplicates]. Uptake in RLIP76-proteoliposomes with ATP is significantly greater than other groups (p<0.005) (panel A). All transport studies were carried out using 250 ng protein/assay except when protein was varied (panel B). Incubation time was 5 min except for time-dependence studies (panel C). Temperature was 37°C except for temperature-dependence studies (panel D). External sucrose concentration was 40 mM, except for studies of osmolarity dependence (panel E). ATP was 4 mM except in ATP-dependence studies (panel F). DNP-SG was 100 μM except in DNPSG-dependence studies (panel G). The proteoliposomes prepared from RLIP76 purified from K562 cells. Mean values ± SD for three experiments are shown.