Abstract
Cyclopentadienone iron alcohol complexes generated from the reactions of [2,5-(SiMe3)2-3,4-(CH2)4(η5-C4COH)]Fe(CO)2H (3) and aldehydes were characterized by 1H NMR, 13C NMR, and IR spectroscopy. The benzyl alcohol complex [2,5-(SiMe3)2-3,4-(CH2)4(η5-C4C=O)]Fe(CO)2(HOCH2C6H5) (6-H) was also characterized by X-ray crystallography. These alcohol complexes are thermally unstable and prone to dissociate the coordinated alcohols. The alcohol ligand is easily replaced by other ligands such as PhCN, pyridine, and PPh3. Dissociation of the alcohol ligand in the presence of H2 leads to the formation of iron hydride 3. The reduction of aldehydes by 3 was carried out in the presence of both potential intermolecular and intramolecular traps. The reaction of 3 with PhCHO in the presence of 4-methylbenzyl alcohol as a potential intermolecular trapping agent initially produced only iron complex 6-H of the newly formed benzyl alcohol. However, the reaction of 3 with 4-(HOCD2)C6H4CHO (11-d2), which possesses a potential intramolecular alcohol trapping agent, afforded two alcohol complexes, one with the newly formed alcohol coordinated to iron and the other with the trapping alcohol coordinated. The intramolecular trapping experiments support a mechanism involving concerted transfer of a proton from OH and hydride from Fe of 3 to aldehydes. The kinetics and mechanism of the hydrogenation of benzaldehyde catalyzed by 3 are presented.
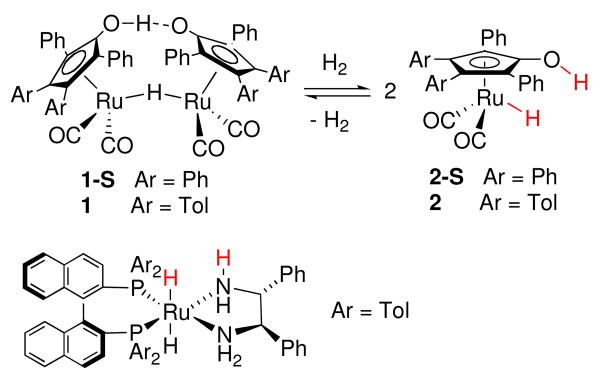
Alcohol complexes play important roles in transition metal catalyzed reactions. They serve as catalyst precursors by dissociating the alcohol ligand and creating vacant coordination sites for substrate binding. They have been proposed as essential intermediates in aerobic alcohol oxidations,i silane alcoholysis,ii and ionic hydrogenation of carbonyl groups.iii Alcohol complexes have been suggested as possible intermediates in the hydrogenation of ketones and aldehydes catalyzed by Shvo's diruthenium hydride complex 1-Siv and its tolyl analog 1. However, all attempts to prepare or spectroscopically observe these ruthenium alcohol complexes have been unsuccessful.v We succeeded in synthesizing related cationic hydroxycyclopentadienyl ruthenium alcohol complexes,vi which dissociate alcohol rapidly at low temperature. Very few alcohol complexes have been crystallographically characterized;iiia,iiie,vii all of the structurally characterized alcohol complexes are cationic compounds.
Ligand-metal bifunctional hydrogenation catalysis is dramatically changing the face of reduction chemistry.viii These transition metal catalysts contain electronically coupled hydridic and acidic hydrogens that are transferred to polar unsaturated species under mild conditions. The first such catalyst, Shvo's (hydroxycyclopentadienyl) diruthenium bridging hydride (1-S), was developed in the mid 1980s.iv,ix,x Noyori has developed a series of chiral ruthenium(diamine)(diphosphine) catalysts, including ruthenium(dpen)(tol-BINAP) (Figure 1), which displays extraordinary activity and enantioselectivity in the hydrogenation of a diverse range of ketones.xi
Figure 1.
Examples of ligand-metal bifunctional catalysts.
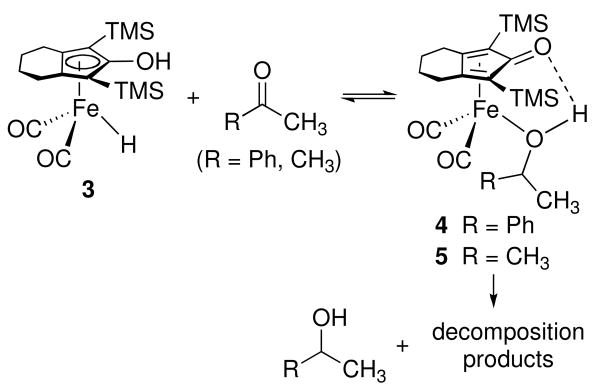
Recently, we discovered that the hydroxycyclopentadienyl iron hydride 3, which is closely related to the active reducing agent in the Shvo ruthenium catalyst system, is an efficient and chemoselective catalyst for the hydrogenation of ketones.xii In the absence of dihydrogen, spectral evidence suggested the formation of an iron alcohol complex: two 1H NMR resonances were seen for the diastereotopic SiMe3 groups of the complex of a chiral alcohol (4), while a single SiMe3 resonance was seen for the complex of a symmetric alcohol complex (5) (Scheme 1). Unfortunately, because the reduction of ketones did not go to completion and because alcohol complexes 4 and 5 slowly decomposed (> 12 h) to unidentified products, it was not possible to isolate and fully characterize these alcohol complexes.
Scheme 1.
Here we describe the synthesis of neutral iron alcohol complexes from complete stoichiometric reduction of aldehydes by iron hydride [2,5-(SiMe3)2-3,4-(CH2)4(η5- C4COH)]Fe(CO)2H (3). We have examined the kinetic and thermodynamic stability of the alcohol complexes toward substitution reactions. We have also studied hydrogen transfer reaction from 3 to aldehydes with both intermolecular and intramolecular alcohol trapping agents. These trapping experiments, in combination with kinetics studies on catalytic aldehyde hydrogenation and alcohol dissociation provide strong evidence that reduction occurs outside the coordination sphere of the metal.
Results
Synthesis and Stability of Iron Alcohol Complexes
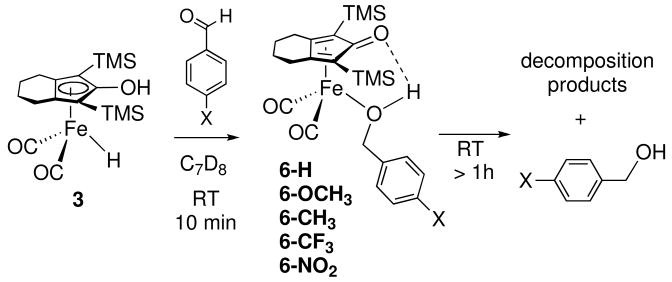
When equimolar amounts of benzaldehyde and [2,5-(SiMe3)2-3,4-(CH2)4(η5-C4COH)]Fe(CO)2H (3) were mixed in toluene-d8, the color of the resulting solution changed from yellow to orange within seconds. After 10 min at room temperature, the 1H NMR spectrum of the solution confirmed the complete reduction of benzaldehyde and the formation of [2,5-(SiMe3)2-3,4-(CH2)4(η5-C4C=O)]Fe(CO)2(HOCH2C6H5) (6-H) in >90% NMR yield. The benzylic hydrogen resonance of 6-H appeared at δ 4.18 (shifted from δ 4.34 in free benzyl alcohol) and the hydrogen bonded hydroxyl resonance was a broad singlet at δ 1.35. Solutions of 6-H in toluene-d8 were stable indefinitely at −30 °C, but underwent significant decomposition within an hour at room temperature. Extended reaction time produced some unidentified iron species as well as the free alcohol. A series of p-substituted benzyl alcohol complexes displayed similar thermal stability (Scheme 2).
Scheme 2.
X-Ray Structure of 6-H
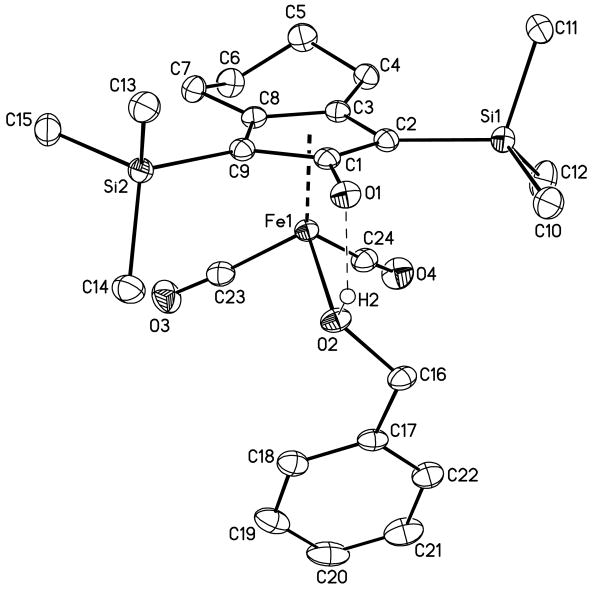
Single crystals of 6-H were grown by slow evaporation of a −30 °C pentane solution of the alcohol complex (generated in situ from hydride 3 and benzaldehyde). In the X-ray crystal structure of 6-H, the Fe center is formally five-coordinate with a bidentate cyclopentadienone ligand, two carbonyls, and an alcohol (Figure 2). The short C(1)–O(1) distance of 1.2768(16) Å is best described as a C=O unit. The five-membered ring of the cyclopentadienone ligand has an envelope shape, with C(1) atom displaced away from the Fe center. The angle between the plane C(2)–C(3)–C(8)–C(9) and the plane C(2)–C(1)–C(9) is 7.7(1)°. As a result, the Fe–C(1) distance of 2.222(1) Å is longer than the distances to C(2) and C(9) (av. 2.126(2) Å), or C(3) and C(8) (av. 2.087(7) Å). O(1) is bent down 10.7(2) toward the alcohol ligand from the C(2)–C(1)–C(9) plane as a consequence of the hydrogen bonding interaction with the complexed alcohol.
Figure 2.
X-ray Crystal Structure of 6-H shown with 50% probability ellipsoids.
Very Rapid Aldehyde Reduction
The rate of reduction of benzaldehyde by 3 in toluene-d8 was too rapid to be determined accurately, even at low temperature. When a solution of 3 (0.009 M) and PhCHO (0.10 M) prepared at -78 °C was inserted into a 1H NMR probe at -72 °C, about 80% reduction had occurred before the first spectrum could be obtained. The rate of the remainder of the reaction was followed to obtain a very approximate rate constant, kobs = 1.6 (7) × 10−3 s-1 (t1/2 ≈ 7 min).xiii For comparison, the rate of reduction of benzaldehyde by ruthenium hydride 2 in toluene was much slower and proceeded at a slower rate even at -37 °C.xiv
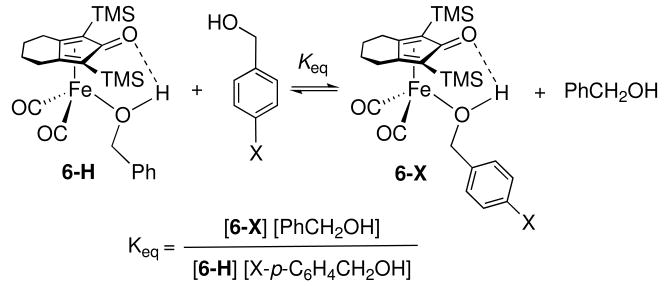
Exchange of Coordinated Alcohols with Free Alcohols
Coordinated alcohols were labile at room temperature and underwent rapid exchange with free alcohols (Scheme 3). Equilibria were established within 10 min.xv More basic alcohols bearing electron-donor substituents preferentially complex to iron (Table 1).
Scheme 3.
Table 1.
| X | σ | Keq (toluene-d8) | Keq (CD2Cl2) |
|---|---|---|---|
| OCH3 | -0.27 | 1.74(28) | 1.49(1) |
| CH3 | -0.17 | 1.35(14) | 1.15(11) |
| CF3 | 0.54 | 0.32(2) | 0.50(3) |
| NO2 | 0.78 | 0.26(8) | 0.35(7) |
Average of equilibrium constants approached from both directions.
Hammett substituent constant, σxvi
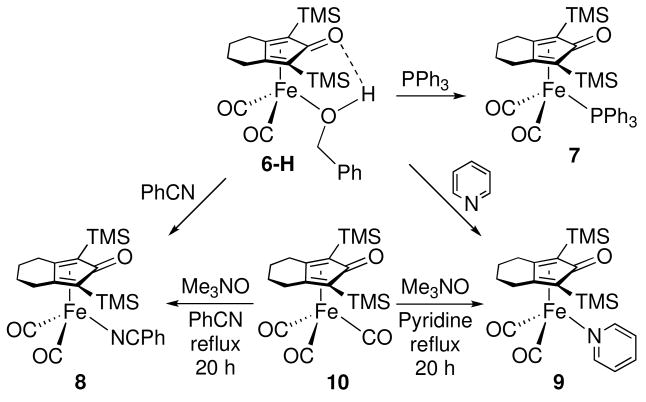
Substitution of Coordinated Alcohol by Triphenylphosphine, Benzonitrile, and Pyridine
Benzyl alcohol was readily displaced from 6-H by other ligands at room temperature. Triphenylphosphine, benzonitrile, and pyridine all reacted rapidly with 6-H to afford the corresponding complexes almost quantitatively (Scheme 4). The PPh3-substituted complex 7 was reported in the literature,xvii and compounds 8 and 9 were synthesized independently.
Scheme 4.
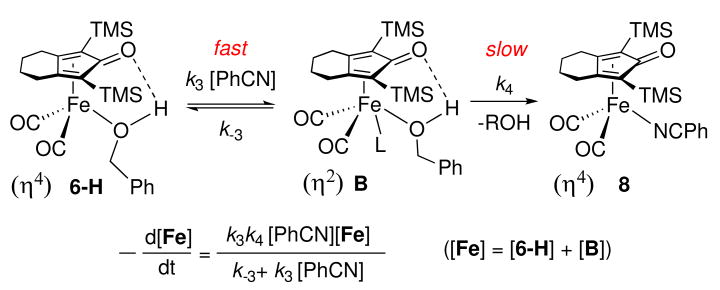
Kinetics of Ligand Substitution of Alcohol Complexes
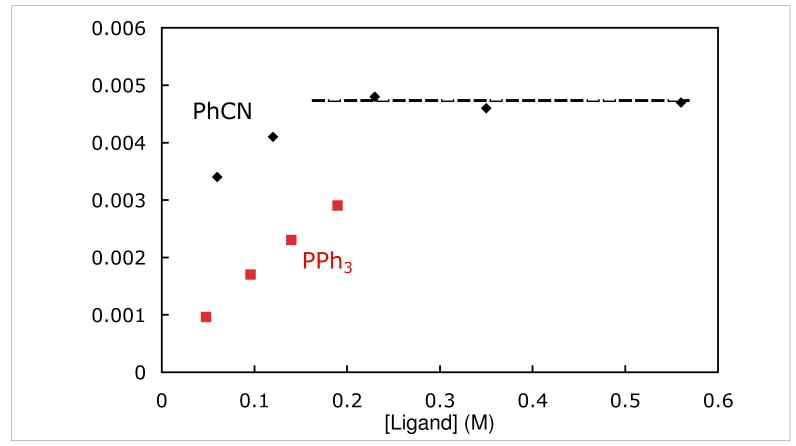
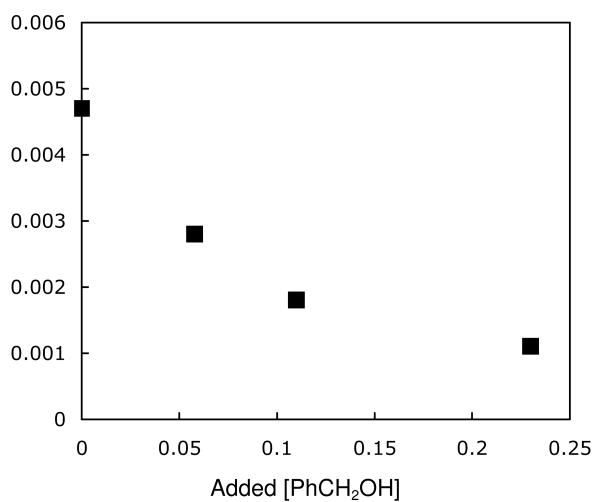
The rate for the substitution of coordinated benzyl alcohol in 6-H by PhCN was studied by 1H NMR spectroscopy. When two equivalents of PhCN (0.060 M) was added to a solution of 6-H (0.030 M) in toluene-d8 at 5 °C, the disappearance of 6-H followed pseudo first order kinetics over more than one half-life. When the concentration of PhCN was increased, kobs for ligand substitution increased and then plateaued above 0.23 M PhCN at a saturation rate of 4.7 (1) × 10-3 s-1 (Figure 3). The effect of added benzyl alcohol on the rate of ligand substitution was measured in the presence of 0.35 M PhCN. Added benzyl alcohol inhibited the rate of ligand substitution (Figure 4).
Figure 3.
Rate of Ligand Substitution of Alcohol from 6-H as a Function of PhCN and PPh3 Concentrations at 5 °C.
Figure 4.
Rate of PhCN Substitution of Alcohol from 6-H as a Function of Added Alcohol Concentration at 5 °C with [PhCN] = 0.35 M.
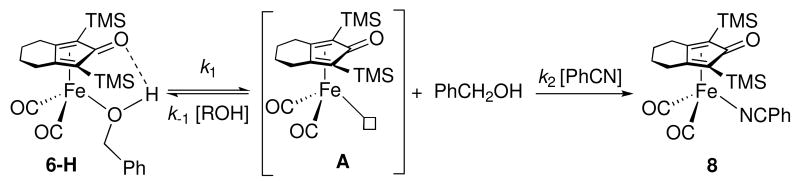
A kinetic mechanism involving reversible dissociation of alcohol followed by PhCN trapping is consistent with these rate measurements (Scheme 5).xviii Assuming steady-state approximation on unsaturated intermediate A, the substitution reaction has the rate law shown in equation (1). At high concentration of PhCN, where k2[PhCN] ≫ k-1[PhCH2OH], the rate law simplifies to equation (2) and the observed rate constant is equal to the alcohol dissociation rate constant k1 (≈ 4.7 × 10-3 s-1). The ratio of k2 / k-1 was estimated to be about 0.21 from the slower rate of reaction in the presence of 0.11 M [PhCH2OH] and 0.35 M [PhCN] {kobs = 1.9 × 10-3 s-1} than in its absence {kobs = 4.7 × 10-3 s-1}.
Scheme 5.
| (1) |
| (2) |
Associative ligand substitution mechanisms involving reversible nitrile coordination concurrent with η4 to η2 ring slippage, followed by irreversible loss of alcohol concurrent with η2 to η4 ring slippage are not consistent with the observed inverse kinetic dependence on alcohol concentration (Scheme 6). Moreover, in an associative mechanism, the observation of saturation kinetics at high nitrile concentration would require significant concentrations of an intermediate. Even if such an intermediate were in rapid equilibrium with the starting alcohol complex, significant changes in the NMR spectrum should have been detected, but none were seen.
Scheme 6.
The temperature dependence of the unimolecular dissociation of alcohol from 6-H was obtained by measuring the saturation rates at 0.35-0.82 M PhCN. Rate constants measured between −10 and 5 °C gave activation parameters ΔH‡ = 15.7 ± 0.8 kcal mol-1 and ΔS‡ = −12.6 ± 3.0 eu.
Complexes of more basic alcohols bearing electron-donor substituents underwent slower alcohol dissociation (Table 2). For example, the rate of dissociation of 4-methoxybenzyl alcohol from 6-OCH3 was about 10 times slower than that of 4-nitrobenzyl alcohol from 6-NO2.
Table 2.
Alcohol Dissociation Rate Constants at 5 °C in Toluene-d8
| Alcohol Complex | σ | k1 × 10-3 (s-1) |
|---|---|---|
| 6-OCH3 | -0.27 | 2.2(1) |
| 6-CH3 | -0.17 | 3.9(1) |
| 6-H | 0.0 | 4.7(1) |
| 6-CF3 | 0.54 | 16(1) |
| 6-NO2 | 0.78 | 20(1) |
The rate for the substitution of coordinated benzyl alcohol in 6-H by PPh3 was slower than that by PhCN at similar ligand concentrations (Figure 3). Moreover, at low [PPh3] (0.048 M), the disappearance of 6-H deviated from an exponential decay. The reaction slowed as the concentration of PhCH2OH built up in solution. These results suggest that PPh3 does not efficiently compete with alcohols for the trapping of intermediate A. In a competition experiment, reaction of 6-H (0.021 M) with excess PPh3 (0.25 M) and PhCN (0.25 M) at 5 °C led to preferential formation of the benzonitrile adduct: 8 : 7 = 2.8 : 1; the ratio of 8 : 7 did not change upon warming to room temperature. A similar competition between PhCN and pyridine showed similar trapping ability of the two ligands 8 : 9 = 0.97 : 1.
Benzaldehyde Reduction in the Presence of 4-Methylbenzyl Alcohol as a Possible Intermolecular Trapping Agent
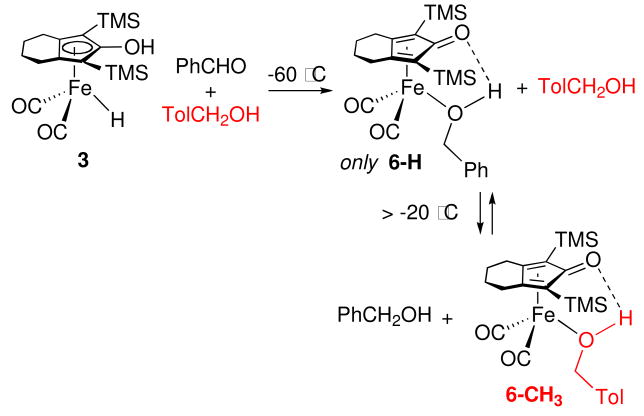
If aldehyde reduction occurs via an outer sphere mechanism, an external trapping agent might intercept unsaturated species A. When the reduction of benzaldehyde by 3 in the presence of 4-methylbenzyl alcohol at −60 °C was monitored by 1H NMR spectroscopy, only iron complex 6-H bearing the newly generated alcohol was observed (Scheme 7). When the solution was warmed to −20 °C, exchange between 6-H and 4-methylbenzyl alcohol began and an equilibrium mixture with 6-CH3 and free benzyl alcohol was seen.
Scheme 7.
Reduction of Aldehydes Possessing a Potential Intramolecular Alcohol Trap
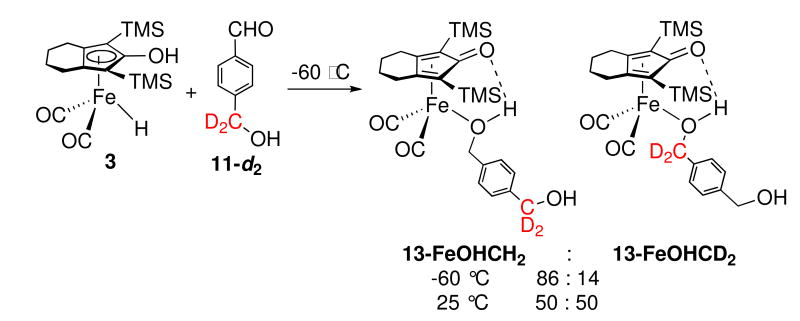
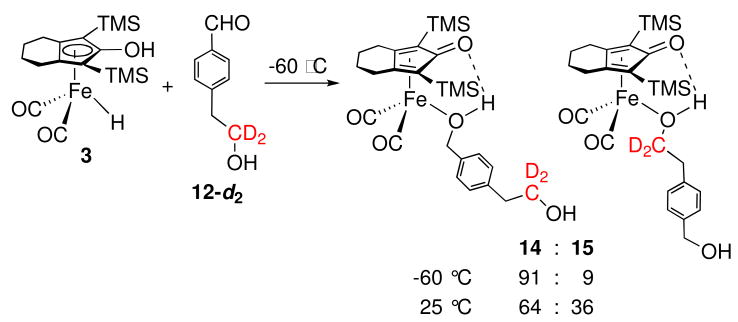
The failure to observe intermolecular trapping is consistent with an inner sphere mechanism leading directly to an alcohol complex and is inconsistent with a simple outer sphere hydrogen transfer mechanism involving free intermediate A. However, the result is consistent with a more restricted outer sphere mechanism where the newly formed alcohol is generated inside a solvent cage and coordinates to iron more rapidly than it dissociates from the cage. The presence of an intramolecular trapping agent might intercept the cage intermediate. Two intramolecular trapping experiments were conducted, one using 4-HOCD2C6H4CHO (11-d2) and the other using 4-HOCD2CH2C6H4CHO (12-d2).
The reaction of 3 with 4-HOCH2C6H4CHO (11) in toluene-d8 at −60 °C gave benzylic alcohol complex 13, whose 1H NMR spectrum had benzylic resonances at δ 3.98 and 4.48 for the coordinated and free benzyl alcohol functionalities, respectively.xix The reduction of deuterated 4-HOCD2C6H4CHO (11-d2) by 3 under the same conditions gave rise to an 86 : 14 ratio of benzylic resonances at δ 3.98 and 4.48. The newly generated alcohol was the major functionality complexed to iron (Scheme 8). This ratio remained constant during the course of reduction and was unchanged between −60 °C and −20 °C. Upon warming above −20 °C, the ratio of the two benzylic resonances began changing, and at 25 °C, a 1 :1 ratio of 13-FeOHCH2 : 13-FeOHCD2 was rapidly approached.xx
Scheme 8.
A similar intramolecular trapping experiment was carried using 12-d2 which has an additional intervening methylene group between the aldehyde and alcohol functionalities. At −60 °C, the reduction of 12-d2 by 3 generated two alcohol products 14 and 15 in a ratio of 91 : 9 with the newly generated alcohol being trapped as the major product (Scheme 9). This kinetic ratio did not change during the course of reduction or upon warming to −20 °C. Upon warming to 25 °C, slow equilibration occurred to give a 64 : 36 mixture of 14 : 15.
Scheme 9.
Reversibility of Aldehyde Reduction
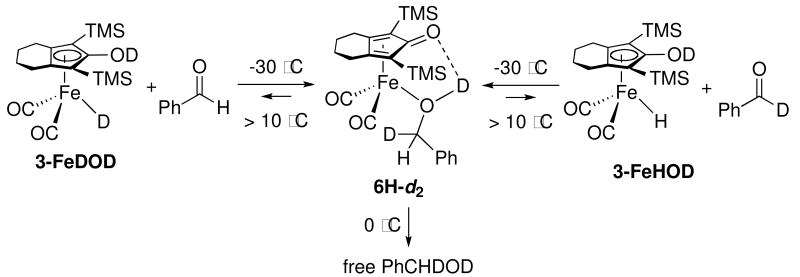
When a solution of dideuterated 3-FeDOD in toluene was treated with 2 equivalents of PhCHO at −30 °C, the 2H NMR spectrum of the mixture confirmed rapid formation of deuterated alcohol complex 6H-d2 (Scheme 10). When the solution was warmed to 0 °C, a small amount of free alcohol PhCHDOD was observed, presumably due to decomposition of the alcohol complex. When the temperature was raised above 10 °C, deuterium incorporation into the excess aldehyde was seen. At room temperature, 40 mol% of the aldehyde was PhCDO. Although neither 3-FeDOD nor 3-FeHOD was detected by 2H NMR once the alcohol complex was generated, the appearance of PhCDO clearly demonstrated that hydrogen transfer was reversible above 10 °C.
Scheme 10.
Stability of Iron Hydride 3
Iron hydride 3 is moderately light sensitive.xxi Exposure of solutions of hydride 3 in toluene-d8 (or CD2Cl2) under a nitrogen atmosphere to ambient fluorescent lighting for several hours led to a color change from yellow to red, but to no appreciable change in the 1H NMR spectra. After more than a day, 1H NMR resonances of 3 became broad, possibly due to the formation of paramagnetic species.
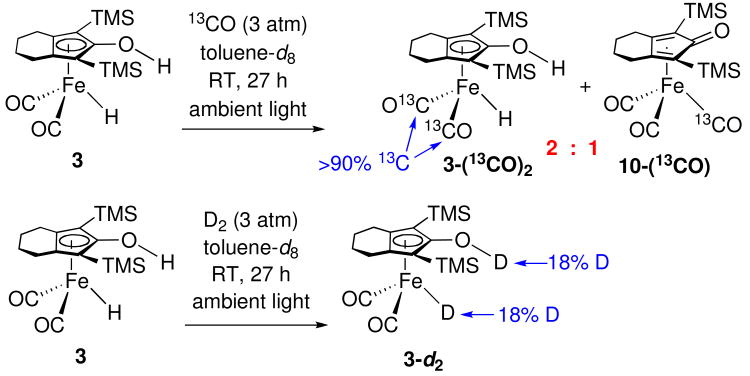
Under ambient fluorescent lighting, toluene-d8 solutions of iron hydride 3 underwent slow exchange with 13CO (Scheme 11). When a 0.04 M solution of 3 in toluene was shaken for 4 h under 3 atm 13CO in ambient light, 1H NMR spectroscopy showed 77% of 3 remained and examination of the 13C splitting of the FeH resonance showed 13CO incorporation: 3 : 3-(13CO) : 3-(13CO)2 = 3 : 2 : 2. In addition, 23 % of 3 was converted to labeled iron tricarbonyl complex 10-(13CO). After 27 h, 66% of the material was 3-(13CO)2 (> 90% label) and 33% 10-(13CO).
Scheme 11.
Under ambient fluorescent lighting, toluene-d8 solutions of iron hydride 3 underwent slow exchange with D2 (Scheme 11). When a 0.04 M solution of 3 in toluene was shaken for 4 h under 3 atm D2 in ambient light, 1H NMR spectroscopy showed somewhat greater incorporation of label into the FeD (21%) than into the OD (9%) position. After 27 h, nearly equal amounts of label in both the FeD (18%) and the OD (18%) positions. For ruthenium hydride 2, we have seen rapid D2 incorporation selectivity into the RuD position at room temperature and much slower incorporation into both the RuD and OD positions at elevated temperature by processes that are still under investigation.xxii
Iron Hydride from Reaction of Alcohol Complex 6-H with H2
The reaction of 6-H under 3 atm H2 pressure at 5 °C was studied by 1H NMR spectroscopy. Iron hydride 3 was the principal product observed; however, some decomposition products were also seen. Attempts to accurately determine the rate of reaction of 6-H with H2 were not successful due to the limited solubility of H2 in toluene-d8 and the inability to rapidly and continuously mix gas and solution inside an NMR probe.
If iron hydride formation proceeds by alcohol dissociation followed by rapid trapping of a reactive intermediate by H2, then the rate of reaction should be similar to the rate of alcohol dissociation. The rate of alcohol dissociation from 6-H was estimated to have a k1 value of 3.4 × 10-2 s-1 (t1/2 ≈ 20 s) at 25 °C, using activation parameters. This dissociation rate is consistent with the rapid rate of reaction with H2 with 6-H.
Hydrogenation of Aldehydes Catalyzed by 3 was studied using an in situ ReactIR apparatus. Analogous to hydrogenation of acetophenone reported earlier,xii the catalytic hydrogenation PhCHO catalyzed by 3 obeyed second order kinetics: first order in both PhCHO and hydride 3, and the hydrogenation rate was not affected by H2 pressure. During hydrogenation, two metal carbonyl absorptions were observed at 2001 and 1941 cm-1; since both iron hydride 3 and alcohol complex 6-H have bands at the same frequencies, it was not possible to determine the nature of the major iron containing species during hydrogenation. The second order rate constant was for hydrogenation of benzaldehyde was determined to be 8.4(1.9) × 10-2 M-1 s-1 at 25 °C.xxii
For comparison, catalytic hydrogenation of acetophenone catalyzed by 3 is about 8 times slower (1.1 × 10-2 M-1 s-1 at 25 °C) than the hydrogenation of benzaldehyde. Ruthenium catalysts 1 ⬄2 and [2,5-Ph2-3,4-Tol2(η5-C4COH)]Ru(CO)(PPh3)H (16) are slower catalystsxxiv but show higher chemoselectivity for reduction of PhCHO over PhCOMe (40 for 1 ⬄2 and 1200 for 16).xxv
Discussion
Alcohol complexes have been suggested as fleeting intermediates in aldehyde and ketone reductions with the Shvo catalyst 1-S ⬄2-S, such alcohol complexes have never been directly observed.v,vi Iron complexes 6 constitute the first examples of neutral alcohol complexes in ligand metal bifunctional catalysis. As in the case of related ruthenium amine complexes, two site binding of the alcohol is observed: the alcohol oxygen is coordinated to iron and the alcohol hydroxyl group is hydrogen bonded to the cyclopentadienone carbonyl. Alcohol complexes 6 are kinetically labile and dissociate at readily measurable rates at 5 °C. Both the kinetic and thermodynamic stability of the alcohol complexes depend on the basicity of the alcohol oxygen. The stronger bases bind more strongly, even though they have weaker hydrogen bonds.
Inner Sphere vs Outer Sphere Reduction Mechanisms
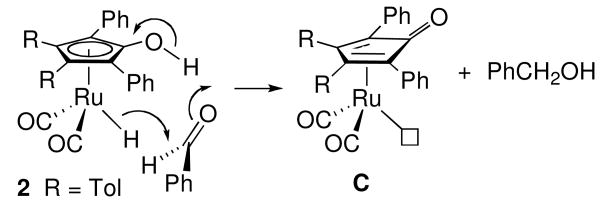
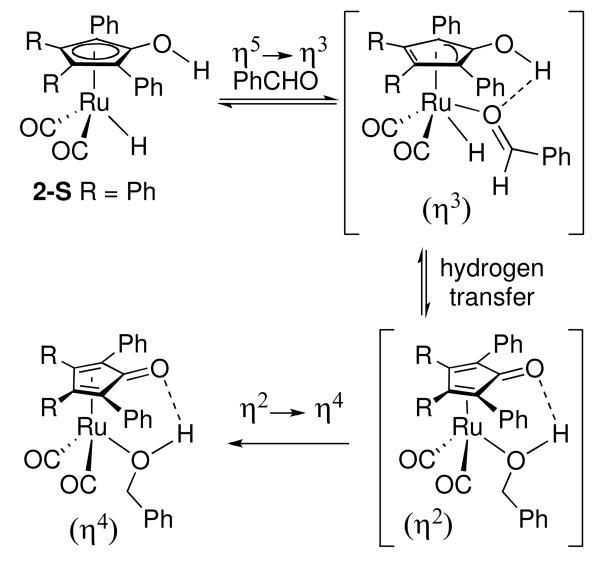
Two mechanisms have been proposed for the reduction of aldehydes by hydroxycyclopentadienyl ruthenium hydride 2, the active reducing species in the Shvo catalytic system. Based on our detailed mechanistic studies of the reduction of benzaldehyde by 2, including observation of primary deuterium isotope effects for transfer of both OH and RuH, we proposed a mechanism involving concerted transfer of proton and hydride to aldehyde outside the coordination sphere of Ru that generates a coordinatively unsaturated intermediate C (Scheme 12).xxvi In our mechanism, an alcohol complex is not necessarily generated during the hydrogenation cycle. Bäckvall has proposed an alternative mechanism that requires coordination of the aldehyde and slippage of the Cp ring prior to hydrogen transfer (Scheme 13).xxvii In this mechanism, the alcohol product is “born” in the coordination sphere of Ru and an alcohol complex is inevitably involved in the catalytic cycle. Previously, it was not possible to distinguish these two mechanisms because alcohol complexes are too labile to be detected.
Scheme 12.
Scheme 13.
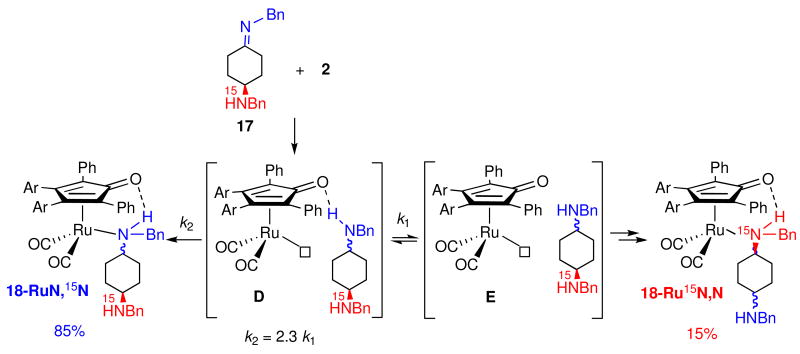
Efforts to distinguish between inner and outer sphere mechanisms turned to the reduction of imines since the resulting amine complexes are kinetically stable at low temperature.xxviii,xxix,xxx The inner sphere mechanism predicts that only the newly formed amine will coordinate to ruthenium, while the outer sphere mechanism, proceeding through coordinatively unsaturated intermediate C, predicts formation of two different amine complexes. The observation that no intermolecular trapping products were formed in the reduction of an imine by 2 in the presence of an external amine trapping agent is consistent with the inner sphere mechanism, and rules out an outer sphere mechanism involving proceeding through an unconstrained coordinatively unsaturated intermediate C. However, the possibility remained that C and the newly formed amine are generated inside a solvent cage and collapse to an amine complex faster than diffusion from the solvent cage. To test this possibility, the reductions of imines containing an intramolecular amine trapping agent were studied. Intramolecular trapping products were observed at low temperature in two of the three cases examined.xxviii,xxix,xxx For example, reduction of 15N-labeled imine 17 gave 15% of the amine complex 18-Ru15N,N resulting from intramolecular amine trapping (Scheme 14).xxx These results were explained in terms of an outer sphere mechanism leading to the coordinatively unsaturated intermediate D which has the initially formed amine hydrogen bonded to the carbonyl oxygen of the dienone. Collapse of D by coordination of nitrogen to give 18-RuN,15N (70%) occurs in competition with cleavage of the hydrogen bond to give caged intermediate E which leads to equal amounts of 18-Ru15N,N (15%) and additional 18-RuN,15N (15%).
Scheme 14.
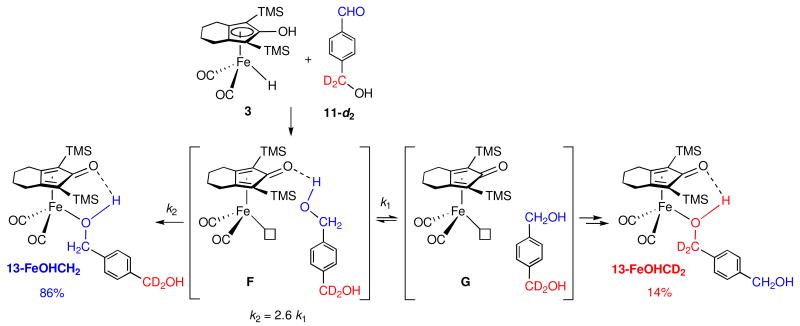
Our synthesis of kinetically stable alcohol complexes in a Shvo type system from the reduction of aldehydes by iron hydride 3 has provided an opportunity to distinguish between inner and outer sphere mechanisms for aldehyde reduction. When the reduction of PhCHO by 3 was carried out in the presence of added 4-methylbenzyl alcohol as a potential intermolecular trapping agent for coordinatively unsaturated intermediate A, only 6-H (the iron complex of the newly generated alcohol) was observed and none of the intermolecular trapping product 6-CH3 was seen. However, two separate intramolecular trapping experiments using alcohol-containing aldehydes 11-d2 and 12-d2 led to the observation of intramolecular trapping. As in the case of imine reduction, we propose an outer sphere mechanism for reduction of aldehyde 11-d2 by 3 leading to the coordinatively unsaturated intermediate F, which has the initially formed alcohol hydrogen bonded to the carbonyl oxygen of the dienone (Scheme 15). Collapse of F by coordination of the alcohol oxygen to give 13-FeOHCH2 (72%) occurs in competition with cleavage of the hydrogen bond to give caged intermediate G which leads to equal amounts of 13-FeOHCD2 (14%) and additional 13-FeOHCH2 (14%).
Scheme 15.
The breaking of the stronger hydrogen bond to an alcohol OH in F is expected to be slower than the breaking of a weaker hydrogen bond to an amine NH in D. Similarly, coordination of the less basic alcohol oxygen in F is expected to be slower than coordination of the more basic nitrogen in D. Apparently, both process for F are slowed by similar amounts relative to those for D; this results in similar ratios of product coordination to cleavage of the hydrogen bond for alcohols (k2 = 2.6 k1) and amines (k2 = 2.3 k1).
Mechanism of Hydrogenation of Aldehydes Catalyzed by 3
We have independently observed many of the likely individual steps in the hydrogenation of aldehydes catalyzed by iron hydride 3. The stoichiometric reduction of benzaldehyde by 3 was rapid at -72 °C and produced iron alcohol complex 6-H. The rate of dissociation of alcohol from 6-H leading to substitution by PhCN or exchange with other alcohols was measured at 5 °C. The rate of reaction of H2 with alcohol complex 6-H to give iron hydride 3 was at least as fast as alcohol dissociation from 6-H.
The rate law for the hydrogenation of benzaldehyde catalyzed by 3 (Rate = k [3][PhCHO][H2]0) is consistent with rate limiting reaction of 3 with PhCHO. The kinetics are consistent with 3 being the major iron species during catalysis, but IR spectroscopy could not distinguish between iron species 3 and 6-H because of the similarity of their spectra and the catalysis was not readily measurable by NMR spectroscopy. While alcohol complex 6-H may be an intermediate in the catalytic process, the extrapolated rate of dissociation of alcohol from 6-H was about 2.5 times slower than the overall catalytic rate; if this rate extrapolation is accurate then 6-H may not be a kinetically viable intermediate.
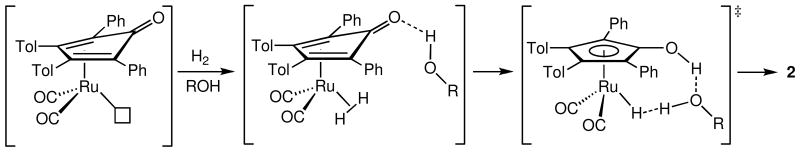
Studies of dihydrogen elimination from hydroxycyclopentadienyl ruthenium hydride 2 demonstrated a key role for alcohols in relaying a proton transfer from the CpOH group to the metal hydride to form an intermediate dihydrogen complex.xxii The microscopic reverse, dihydrogen activation, was therefore proposed to require an alcohol also (Scheme 16). Similar mechanisms for dihydrogen activation are likely for the iron systems reported here.
Scheme 16.
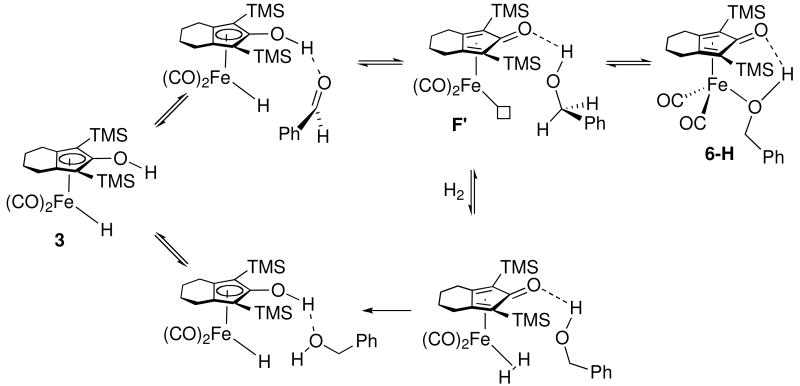
Several possible detailed mechanisms for hydrogenation of benzaldehyde catalyzed by 3 are summarized in Scheme 17. One possibility is that alcohol complex 6-H is an intermediate in the catalysis and that it dissociates alcohol faster than its extrapolated rate. A second possibility is that 6-H is an intermediate and that it reacts with H2 faster than alcohol fully dissociates; dihydrogen addition may occur to F', a coordinatively unsaturated intermediate which retains a hydrogen from the alcohol to the cyclopendienone carbonyl. A third possibility is that F' is generated from reaction of PhCHO with 3 and reacts with dihydrogen faster than it collapses to alcohol complex 6-H. Further experiments are needed to sort out these possibilities.
Scheme 17.
Experimental Section
Synthesis of Alcohol Complexes
For the convenience of compound characterization, alcohol complexes were generated in situ by mixing equimolar amounts of hydride 3 (12 μmol) and an aldehyde (12 μmol) in 500 μL of CD2Cl2 (or toluene-d8) in a resealable NMR tube for 10 min at room temperature. NMR spectra of the orange alcohol complex solutionsxxxi were obtained in an NMR probe cooled to 0 °C. Infrared spectra of the alcohol complexes (in toluene) were taken in a CaF2 solution cell at room temperature.
{2,5-(SiMe3)2-3,4-[(CH2)4](η4-C4CO)}Fe(CO)2(HOCH2Ph) 6-H)
1H NMR (CD2Cl2, 360 MHz, 0 °C) δ 0.20 (s, Si(CH3)3, 18H), 1.25-1.29 (m, CHH, 2H), 1.43-1.48 (m, CHH, 2H), 1.98-2.12 (m, CH2, 4H), 4.53 (s, PhCH2OH, 2H), 7.31-7.43 (m, Ar, 5H), OH resonance was not located. 1H NMR (toluene-d8, 360 MHz, 0 °C) δ 0.38 (s, Si(CH3)3, 18H), 0.95-1.12 (m, CH2, 4H), 1.47 (s, OH, 1H), 1.76-1.89 (m, CHH, 2H), 1.90-2.03 (m, CHH, 2H), 4.14 (s, PhCH2OH, 2H), 7.09-7.20 (m, Ar, 5H). 13C{1H} NMR (CD2Cl2, 90 MHz, 0 °C) δ 0.28 (Si(CH3)3), 22.26 (CH2), 24.81 (CH2), 73.70 (PhCH2OH), 75.57, 107.38, 128.82 (CH), 128.98 (CH), 129.02 (CH), 138.24 (CCH2OH), 170.99 (C=O), 212.63 (Fe(CO)2). 13C{1H} NMR (toluene-d8, 90 MHz, 0 °C) δ 0.39 (Si(CH3)3), 22.02 (CH2), 24.69 (CH2), 73.21 (PhCH2OH), 75.63, 107.20, 171.64 (C=O), 212.75 (Fe(CO)2); aromatic carbon resonances were not located. IR (toluene) 1998, 1939 cm-1.
{2,5-(SiMe3)2-3,4-[(CH2)4](η4-C4CO)}Fe(CO)2(PhCN) (8)
PhCN (200 μL, 2.0 mmol) and Me3NO (90 mg, 1.2 mmol) were sequentially added to a solution of iron tricarbonyl complex 10 (418 mg, 1.0 mmol) in acetone (40 mL). The resulting orange solution was refluxed under N2 for 20 h. After cooling, H2O (50 mL) was added and the mixture was extracted with CH2Cl2 (3 × 20 mL). The extract was dried (Na2SO4) and solvent was evaporated under vacuum. The residue was purified by column chromatography (1:1 CH2Cl2 : hexanes, then 1:4 EtOAc : hexanes) to give 8 as an orange powder (451 mg, 91% yield). 8 is not air sensitive, but is moderately light sensitive. 1H NMR (CDCl3, 300 MHz) δ 0.23 (s, Si(CH3)3, 18H), 1.50-1.73 (m, CH2, 4H), 2.24-2.47 (m, CH2, 4H), 7.42-7.53 (m, Ar, 2H), 7.54-7.64 (m, Ar, 1H), 7.77-7.84 (m, Ar, 2H). 13C{1H} NMR (CDCl3, 90 MHz) δ 0.19 (Si(CH3)3), 22.59 (CH2), 25.14 (CH2), 70.94, 107.22, 112.31, 128.13, 129.33 (CH), 133.05 (CH), 133.37 (CH), 180.12 (C=O), 213.03 (Fe(CO)2). IR (CH2Cl2) 2000, 1942, 1585 cm-1. HRMS (ESI) calcd (found) for [C24H31O3FeNSi2+H]+ 492.1317 (492.1335).
4-HOCD2C6H4CHO (11-d2) was prepared by hydrolysis of 4-HOCD2C6H4CH(OCH3)2, which was synthesized by reducing 4-(CHO)C6H4CH(OCH3)2 with LiAlD4.xxxii 1H NMR showed 99% deuteration at the benzylic position.
4-HOCD2CH2C6H4CHO (12-d2) was prepared from 4-BrC6H4CH2CD2OH (which was synthesized by reducing 4-BrC6H4CH2COOH with LiAlD4) according to a similar procedure reported in the literature.xxxiii 1H NMR showed 99% deuteration at the CD2OH position.
Intermolecular Trapping Experiment
A solution of 3 (7.8 mg, 20 μmol) and about 1 equiv of 4-methylbenzyl alcohol in 400 μL of toluene-d8 in a Teflon capped NMR tube was frozen in a liquid nitrogen bath. The frozen solution was layered with 100 μL of toluene-d8 first, and then a layer of aldehyde (20 μmol) in toluene-d8 (100 μL). The mixture was thawed in a dry ice/Et2O bath (−100 °C) and the three layers were carefully mixed while cold. The NMR tube was then immediately inserted into an NMR spectrometer precooled to −60 °C, and spectra were acquired.
Intramolecular Trapping Experiments
A solution of 3 (7.8 mg, 20 μmol) in 400 μL of toluene-d8 in a Teflon capped NMR tube was frozen in a liquid nitrogen bath. The frozen solution was layered with 100 μL of toluene-d8 first, and then a layer of hydroxyaldehyde 11-d2 or 12-d2 (20 μmol) in toluene-d8 (100 μL). The mixture was thawed in a dry ice/Et2O bath (−100 °C) and the three layers were carefully mixed while cold. The NMR tube was then immediately inserted into an NMR spectrometer precooled to −60 °C, and spectra were acquired.
Supplementary Material
Acknowledgments
Financial support from the Department of Energy, Office of Basic Energy Sciences is gratefully acknowledged. Grants from NSF (CHE-9208463 and CHE-9709065) and NIH (1 S10 RR0 8389-01) for the purchase of NMR spectrometers are acknowledged. We thank Dr. Ilia Guzei for assistance with X-ray crystallography and Dr. Charles Fry for assistance with NMR spectrometry.
Footnotes
Supporting Information Available: General experimental information, experimental procedures, synthesis of alcohol complexes, rates of substitution of coordinated alcohols by PhCN, rates of hydrogenation of aldehydes catalyzed by 3, X-ray crystallographic data for {2,5-(SiMe3)2-3,4-[(CH2)4](η4-C4CO)}Fe(CO)2(HOCH2Ph) (6-H). This material is available free of charge via the Internet at http://pubs.acs.org.
References
- i.Sigman MS, Jensen DR. Acc Chem Res. 2006;39:221. doi: 10.1021/ar040243m. [DOI] [PubMed] [Google Scholar]
- ii.Luo XL, Crabtree RH. J Am Chem Soc. 1989;111:2527. [Google Scholar]
- iii.(a) Song JS, Szalda DJ, Bullock RM, Lawrie CJC, Rodkin MA, Norton JR. Angew Chem Int Ed. 1992;31:1233. [Google Scholar]; (b) Smith KT, Norton JR, Tilset M. Organometallics. 1996;15:4515. [Google Scholar]; (c) Bakhmutov VI, Vorontsov EV, Antonov DY. Inorg Chim Acta. 1998;278:122. [Google Scholar]; (d) Bullock RM, Voges MH. J Am Chem Soc. 2000;122:12594. [Google Scholar]; (e) Song JS, Szalda DJ, Bullock RM. Organometallics. 2001;20:3337. [Google Scholar]; (f) Voges MH, Bullock RM. J Chem Soc Dalton Trans. 2002:759. [Google Scholar]
- iv.Blum Y, Czarkie D, Rahamim Y, Shvo Y. Organometallics. 1985;4:1459. [Google Scholar]
- v.Casey CP, Bikzhanova GA, Bäckvall JE, Johansson L, Park J, Kim YH. Organometallics. 2002;21:1955. [Google Scholar]
- vi.Casey CP, Vos TE, Bikzhanova GA. Organometallics. 2003;22:901. [Google Scholar]
- vii.(a) Milke J, Missling C, Sünkel K, Beck W. J Organomet Chem. 1993;445:219. [Google Scholar]; (b) Kimblin C, Bridgewater BM, Churchill DG, Parkin G. Chem Commun. 1999:2301. doi: 10.1021/ic000093l. [DOI] [PubMed] [Google Scholar]
- viii.For reviews of ligand-metal bifunctional catalysis, see: Noyori R, Ohkuma T. Angew Chem Int Ed. 2001;40:40–73.Noyori R, Kitamura M, Ohkuma T. Proc Natl Acad Sci USA. 2004;101:5356–5362. doi: 10.1073/pnas.0307928100.Clapham SE, Hadzovic A, Morris RH. Coord Chem Rev. 2004;248:2201–2237.Ikariya T, Murata K, Noyori R. Org Biomol Chem. 2006;4:393–406. doi: 10.1039/b513564h.
- ix.Shvo Y, Czarkie D, Rahamim Y, Chodosh DF. J Am Chem Soc. 1986;108:7400. [Google Scholar]
- x.(a) Menashe N, Shvo Y. Organometallics. 1991;10:3885. [Google Scholar]; (b) Menashe N, Salant E, Shvo Y. J Organomet Chem. 1996;514:97. [Google Scholar]
- xi.(a) Noyori R, Ohkuma T. Pure Appl Chem. 1999;71:1493. [Google Scholar]; (b) Doucet H, Ohkuma T, Murata K, Yokozama T, Kozawa M, Katayama E, England AF, Ikariya T, Noyori R. Angew Chem Int Ed. 1998;37:1703. doi: 10.1002/(SICI)1521-3773(19980703)37:12<1703::AID-ANIE1703>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- xii.Casey CP, Guan H. J Am Chem Soc. 2007;129:5816. doi: 10.1021/ja071159f. [DOI] [PubMed] [Google Scholar]
- xiii.Assuming second order kinetics, this corresponds to a second order rate constant of 1.6 (7) × 10-2 M-1 s-1 at −72 °C in toluene-d8.
- xiv.The rate constant for reduction of benzaldehyde by 2 was extrapolated to −72 °C using activation parameters: 1.2 × 10-4 M-1 s-1. Casey CP, Johnson JB. Can J Chem. 2005;83:1339.
- xv.Although some decomposition process occurred to alcohol complexes when mixed with free alcohols, the equilibrium constants were not affected.
- xvi.Hammett LP. Physical Organic Chemistry. 2nd. McGraw-Hill; New York: 1970. p. 356. [Google Scholar]
- xvii.Pearson AJ, Shively RJ, Jr, Dubbert RA. Organometallics. 1992;11:4096. [Google Scholar]
- xviii.A more detailed molecular mechanism involving dissociation of alcohol in a two-step process, first breaking of Fe-O bond then breaking the hydrogen bond is also consistent with the observed kinetics.
- xix.Based on the 1H NMR spectra of 6-H, resonances at δ 3.98 and 4.48 were assigned to CH2 next the coordinated and uncoordinated OH's, respectively.
- xx.At 25 °C, a third alcohol complex (δ 4.14) along with the free alcohol was seen. This product was assigned as a diiron alcohol complex with the alcohol ligand bridging two iron centers.
- xxi.The solid sample of 3 is also moderately light sensitive. Compound 3 is best stored in an amber vial and placed in a −30 °C glovebox freezer.
- xxii.Casey CP, Johnson JB, Singer SW, Cui Q. J Am Chem Soc. 2005;127:3100. doi: 10.1021/ja043460r. [DOI] [PubMed] [Google Scholar]
- xxiii.Similarly, iron catalyzed hydrogenation of 4-tolualdehyde gave a second order rate constant of 1.1(1) × 10-1 M-1 s-1 at 25 °C.
- xxiv.The rate of acetophenone hydrogenation catalyzed by the ruthenium analog of 3 is similar to that of 3. The faster rates for both 3 and its Ru analog are attributed to the presence of only monometallic species. This will be detailed in a future publication.
- xxv.Casey CP, Strotman NA, Beetner SE, Johnson JB, Priebe DC, Guzei IA. Organometallics. 2006;25:1236. [Google Scholar]
- xxvi.Casey CP, Singer SW, Powell DR, Hayashi RK, Kavana M. J Am Chem Soc. 2001;123:1090. doi: 10.1021/ja002177z. [DOI] [PubMed] [Google Scholar]
- xxvii.Csjernyik G, Éll AH, Fadini L, Pugin B, Bäckvall JE. J Org Chem. 2002;67:1657. doi: 10.1021/jo0163750. [DOI] [PubMed] [Google Scholar]
- xxviii.Casey CP, Bikzhanova GA, Cui Q, Guzei IA. J Am Chem Soc. 2005;127:14062. doi: 10.1021/ja053956o. [DOI] [PubMed] [Google Scholar]
- xxix.Samec JSM, Éll AH, Åberg JB, Privalov T, Eriksson L, Bäckvall JE. J Am Chem Soc. 2006;128:14293. doi: 10.1021/ja061494o. [DOI] [PubMed] [Google Scholar]
- xxx.Casey CP, Clark TB, Guzei IA. J Am Chem Soc. 2007;129:11821. doi: 10.1021/ja073370x. [DOI] [PubMed] [Google Scholar]
- xxxi.The solution of 6-NO2 in toluene-d8 had a deep purple color.
- xxxii.For reaction procedures, see: Creary X, Hartandi K. J Phys Org Chem. 2001;14:97.
- xxxiii.Ackerley N, Brewster AG, Brown GR, Clarke DS, Foubister AJ, Griffin SJ, Hudson JA, Smithers MJ, Whittamore PRO. J Med Chem. 1995;38:1608. doi: 10.1021/jm00010a005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.