Table 1.
Triazolium catalyzed annulations of enals and alpha-hydroxyenones.a
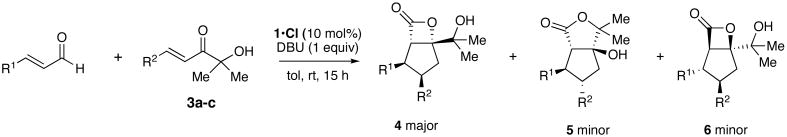 | |||||
|---|---|---|---|---|---|
| entry | R1 = | R2 = | % yieldb | 4:5:6c | % eed |
| 1 (a) | Ph | CO2Et (3a) | 95 (65) | 7e:2:1 | 99;88;99 |
| 2 (b) | p-MeOC6H4 | CO2Et (3a) | 90 (60) | 6:2:1 | 99 |
| 3 (c) | p-BrC6H4 | CO2Et (3a) | 91 (62) | 6:2f:1 | 99 |
| 4 (d) | p-CF3C6H4 | CO2Et (3a) | 89 (35) | 2:1:2 | 99 |
| 5 (e) | 1-naphthyl | CO2Et (3a) | (45) | nd | 99 |
| 6 (f) | 2-furyl | CO2Et (3a) | (40) | nd | 99 |
| 7 (g) | Ph | p-BrC6H4 (3b) | 73 (50) | –:3f:1f | –;93;99 |
| 8 (h) | Ph | p-CF3C6H4 (3c) | 91 (71) | –:4:1 | –;92;99 |
See Supporting Information for reaction details.
Total isolated yields of lactone products; yields of the major product in parenthesis
Determined by 1H NMR analysis of unpurified reaction mixtures.
Enantiomeric excesses of 4,5,6 as determined by SFC analysis on chiral columns. The ee of the minor products in entries 2–7 were not determined.
Structure determined by X-ray analysis of the corresponding methyl ester.
Structure determined by X-ray analysis.
