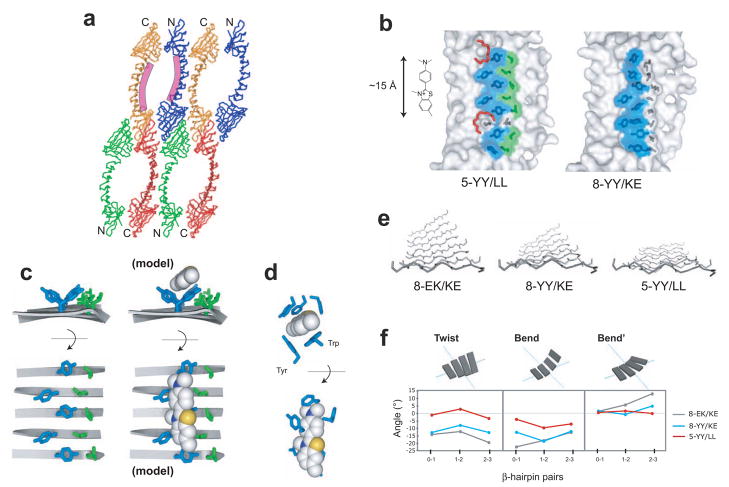
Figure 5.
Crystal structures of ThT-binding PSAMs. (a) Packing of 5-YY/LL monomers in the crystal creates large solvent-channels. The location of the designed ThT-binding site is shown as the purple curves along the central β-sheet of two PSAMs. The N- and C-terminal caps are denoted. (b) Structures of 5-YY/LL and 8-YY/KE. Only the central β-sheet of each PSAM is shown, drawn to scale with ThT. The side chains of Ladder1 and Ladder2 are shown as sticks. The ladder mutations and their solvent-exposed surfaces are colored blue for Tyr and green for Leu, while wild-type side chains are in dark grey. The two PEG-400 molecules found near the putative ThT-binding site on 5-YY/LL are shown as red sticks. (c) The side chain conformations of the 5-YY/LL ladders. The ladders are shown looking down the long axis of the PSAM from C- to N-terminus (top) and looking straight down on the SLB (bottom). The backbone is depicted using ribbons, with turns omitted for clarity. At right, a model of ThT bound to the Tyr surface in an orientation consistent with our results is shown using the same depiction. The ThT ring conformation is derived from that observed in acetylcholinesterase. (d) The ThT-binding pocket of acetylcholinesterase shown in orthogonal views (PDB 2J3Q).24 All residues within 4 Å of ThT are shown as sticks, except for Tyr 121, which is omitted for clarity. The Tyr and Trp residues forming the orthogonal binding motif similar to that observed in 5-YY/LL are labeled (top). (e) The backbone conformations of PSAMs. The eight-stranded β-sheet used as the host in this work is shown looking from the C- to N-terminus, with turns omitted for clarity. The two ladders would be located above the β-sheet as shown. (f) Values of the three parameters Twist, Bend, Bend’ describing the 3-dimensional rotations of each β-hairpin in the SLB.25 The types of rotations are schematically shown above the graphs. Zero values define a perfectly flat, rectangular β-sheet.