Table 2.
Nucleophile screen with BF3•OEt2
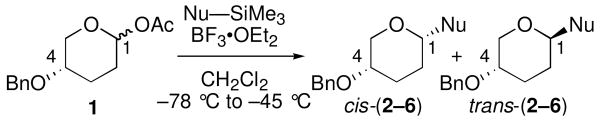 | |||||
|---|---|---|---|---|---|
| entry | Nu–SiMe3 | Na | product | cis:transb,c | yield (%)d |
| 1 | 7 | 1.8 | 2 | 8:92 | 82 |
| 2 | 8 | 6.2 | 3 | 8:92 | 87 |
| 3 | 9 | 8.2 | 4 | 50:50 | 88 |
| 4 | 10 | 9.0 | 5 | 58:42 | 80 |
| 5 | 11 | 10.2 | 6 | 60:40 | 86 |
N = nucleophilicity parameter; see ref 17.
Determined by GC and 1H NMR spectroscopic analysis of the unpurified reaction mixture.
Diastereoselectivities were independent of starting anomer ratio.
Isolated yield.
