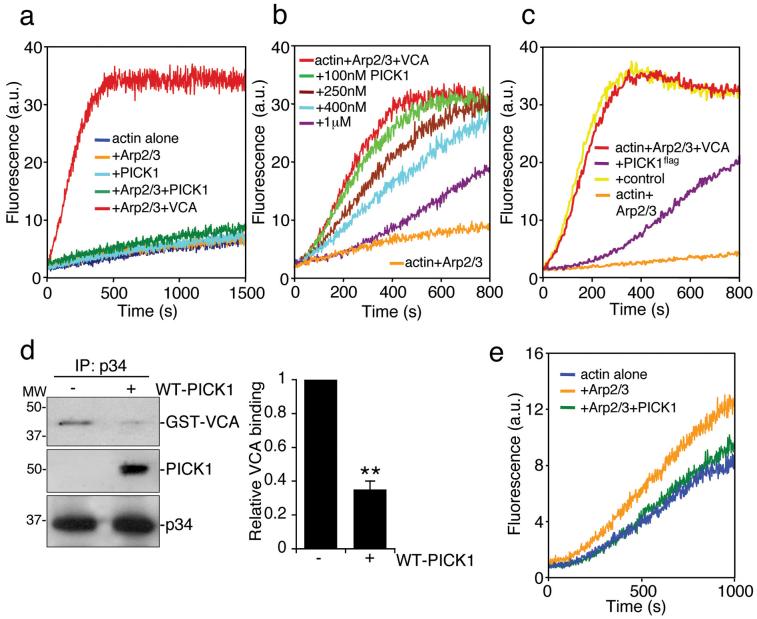
Figure 2.
PICK1 inhibits VCA and Arp2/3-mediated actin polymerisation.
(a) PICK1 does not activate the Arp2/3 complex. In vitro polymerisation of pyrene-labelled actin (2.5 μM) was monitored using time-based fluorimetry. Additional components were: 25 nM Arp2/3 complex, 100 nM GST-VCA, 1 μM his6PICK1.
(b) PICK1 inhibits VCA and Arp2/3-stimulated actin polymerisation in a dose-dependent manner. Various concentrations of his6PICK1 (0.1 – 1 μM) were added to 2.5 μM pyrene-actin, 25 nM Arp2/3 and 100 nM GST-VCA in an in vitro polymerisation assay.
(c) PICK1 purified from neurons inhibits VCA and Arp2/3-stimulated actin polymerisation. High-density cortical neurons were transduced with PICK1flag-IRES-EGFP Sindbis virus. PICK1flag was purified by immunoprecipitation (see methods). Proteins eluted with the flag peptide were added to pyrene-actin, Arp2/3 complex and GST-VCA in an in vitro polymerisation assay. Eluates of immunoprecipitations from non-transduced neurons were used as control.
(d) PICK1 and VCA compete for binding to the Arp2/3 complex. Purified Arp2/3 was immunoprecipitated with anti-p34 antibody. Immunocomplexes were incubated with 100 nM purified GST-VCA with or without 500 nM his6PICK1. After washing of beads, proteins were detected by immunoblotting. Left panel shows representative western blots, right panel shows quantification of relative GST-VCA binding to Arp2/3 in the absence or presence of PICK1. n=3 **p<0.01. Data are means +/− SEM.
(e) PICK1 inhibits the Arp2/3 complex directly. Polymerisation of 5 μM pyrene-labelled actin in the presence of 100 nM Arp2/3 was inhibited by 400 nM his6PICK1.