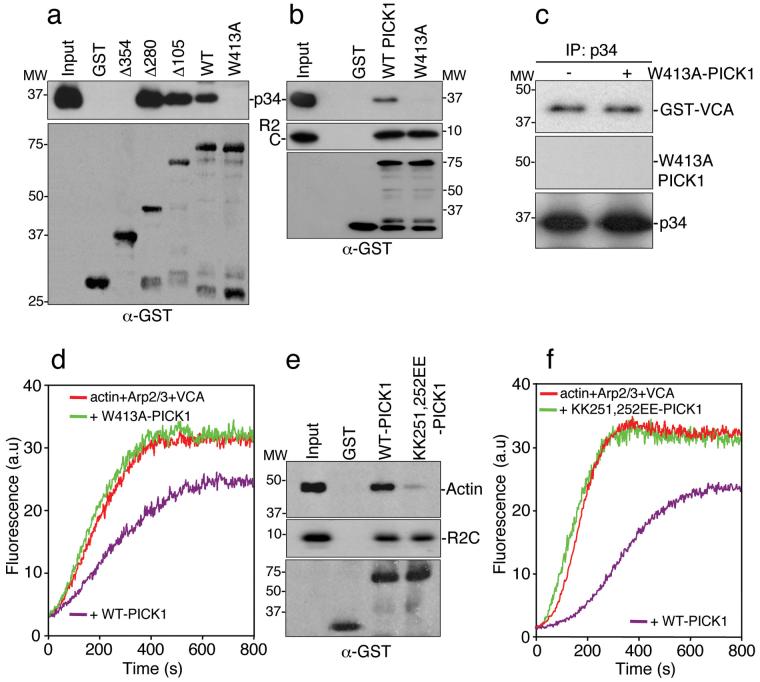
Figure 3.
Identification of functional Arp2/3 and actin binding sites on PICK1.
(a) Amino acids 280-416 are the minimal region for Arp2/3 binding to PICK1. GST fusions corresponding to the stated regions of PICK1 (see Supplementary Information, Fig. S1 online) were immobilised on beads and incubated with 10 nM Arp2/3 complex. Bound proteins were detected by immunoblotting.
(b) Tryptophan 413 is required for Arp2/3 binding. GST-WT-PICK1 and GST-W413A-PICK1, in which the tryptophan residue is mutated to alanine, were immobilised on beads and incubated with 10 nM Arp2/3 complex or 10 nM his6-myc-tagged GluR2 C-terminus (his6mycR2C). Bound proteins were detected by immunoblotting.
(c) W413A-PICK1 does not compete with VCA for binding to Arp2/3 complex. Purified Arp2/3 was immunoprecipitated with anti-p34 antibody. Immunocomplexes were incubated with 100 nM GST-VCA with or without 500 nM his6W413A-PICK1. Bound proteins were detected by immunoblotting.
(d) W413A-PICK1 does not inhibit actin polymerisation. In vitro polymerisation of 2.5 μM pyrene-labelled actin in the presence of 25 nM Arp2/3, 100 nM GST-VCA and 400 nM his6WT-PICK1 or his6W413A-PICK1.
(e) Lysines 251, 252 are required for actin binding. GST-WT-PICK1 and GST-KK251,252EE-PICK1 were immobilised on beads and incubated with in vitro-polymerised F-actin (from 1 μM purified G-actin) or 10 nM his6-myc-tagged GluR2 C-terminus (his6mycR2C). Bound protein was detected by immunoblotting.
(f) KK251,252EE-PICK1 does not inhibit actin polymerisation. In vitro polymerisation of 2.5 μM pyrene-labelled actin in the presence of 25 nM Arp2/3, 100 nM GST-VCA and 400 nM his6WT-PICK1 or his6KK251,252EE-PICK1.