ShuA from S. dysenteriae was crystallized in several crystallization conditions containing detergents. Adding heavy atoms during crystallization strongly improved the crystal quality and the resolution limits. Diffraction data were collected at an energy remote from the Pb M absorption edges.
Keywords: ShuA, Shigella dysenteriae, TonB-dependent haem outer membrane transporters
Abstract
As part of efforts towards understanding the crystallization of membrane proteins and membrane transport across the outer membrane of Gram-negative bacteria, the TonB-dependent haem outer membrane transporter ShuA of Shigella dysenteriae bound to heavy atoms was crystallized in several crystallization conditions using detergents. The insertion of a His6 tag into an extracellular loop of ShuA, instead of downstream of the Escherichia coli peptide signal, allowed efficient targeting to the outer membrane and the rapid preparation of crystallizable protein. Crystals diffracting X-rays beyond 3.5 Å resolution were obtained by co-crystallizing ShuA with useful heavy atoms for phasing (Eu, Tb, Pb) by the MAD method at the synchrotron, and the SAD or SIRAS method at the Cu wavelength. The authors collected X-ray diffraction data at 2.3 Å resolution using one crystal of ShuA-Pb, and at 3.2 Å resolution at an energy remote from the Pb M absorption edges for phasing on PROXIMA-1 at SOLEIL.
1. Introduction
The capability to scavenge essential nutrients, including iron, is essential for the multiplication and virulence of pathogenic Gram-negative bacteria. Although iron is a major component of the Earth’s crust, its bioavailability is very low (Neilands, 1995 ▶); under aerobic conditions or at physiological pH it precipitates to form insoluble ferric oxyhydroxide complexes. Its free concentration in biological fluids is very low (10−18 M) and is less than that required for bacterial growth (10−7–10−8 M; Stintzi et al., 2000 ▶). In humans, iron is bound to proteins such as lactoferrin, ferritin or haemoglobin. In these environments, most pathogenic Gram-negative bacteria are able to use iron after the synthesis and release into the external medium of iron chelators called siderophores. These bind iron(III) with high affinity and are specifically transported across the outer membrane by TonB-dependent transporters (TBDTs; Krewulak & Vogel, 2008 ▶). Many bacterial species such as Vibrio cholerae, Yersinia pestis, pathogenic Escherichia coli (for example, strain O157:H7) and Shigella dysenteriae express haem TBDTs and are therefore able to use free haem or haem from haemoglobin as an iron source (Paulley et al., 2007 ▶). Other organisms such as Serratia marcescens secrete proteins called haemophores which bind to haem and are recognized by their respective TBDT for haem transport (Cescau et al., 2007 ▶).
Shigella dysenteriae is a human pathogenic bacterium belonging to the Enterobacteriaceae and is mainly responsible for dysentery. As with other human pathogenic bacteria, it is able to acquire the iron necessary for growth from haem by using its TonB-dependent haem outer membrane transporter ShuA, which binds to human haemoglobin to transfer haem across the outer membrane (Mills & Payne, 1997 ▶; Burkhard & Wilks, 2007 ▶). ShuA can either bind to haem or to haemoglobin for haem import. The energy required for the transport is derived from the proton motive force and is transferred to the TBDT by the energy-transducing complex TonB–ExbB–ExbD of the inner membrane. Energy is delivered after interactions between the TonB protein and a conserved N-terminal sequence called the TonB-box of the TBDT (Ferguson & Deisenhofer, 2002 ▶).
His6-tagged ShuA was overexpressed in E. coli and purified using a two-step purification. Crystals were obtained in several crystallization conditions. The insertion of the His6 tag in an extracellular loop (Ferguson et al., 1998 ▶; Brillet et al., 2009 ▶), instead of after the signal peptide, is a valuable strategy for overexpression and purification and does not prevent crystallization. Our spectroscopic studies showed that ShuA was able to bind haem in detergent micelles, even with the insertion of the His6 tag in an extracellular loop. By cocrystallizing ShuA with positively charged heavy ions such as Pb2+, Eu3+ and Tb3+, which have a strong anomalous signal at the Cu wavelength for SAD phasing, the size and quality of the ShuA crystals were improved. Crystals of ShuA-Pb that diffracted X-rays to 2.3 Å resolution were obtained and a complete data set was collected at 2.6 Å resolution. X-ray data were also collected to 3.2 Å resolution at an energy remote from the Pb M absorption edges for phasing.
2. Material and methods
2.1. Cloning
The DNA encoding ShuA (without the 28-residue signal sequence) was amplified by PCR. The forward primer of the first fragment F1 (5′-GTCAGATATC ACTGAAACCATG-3′) contained an EcoRV site (underlined) and codons for residues 29–32 (bold). The reverse primer (5′-GCTAGGATCC TTGTTCCTGACGATAA-3′) for F1 contained a BamHI site (underlined) and anticodons for residues 348–352 (bold). The forward primer of the second fragment F2 (5′-GATCGGATCC CATCACCATCATCACCAT CATCCGGGCGGC-3′) contained a BamHI site (underlined) that introduces two residues GS, a DNA sequence encoding a His6 tag (italicized) and codons for residues 353–356. The reverse primer of F2 (5′-GATCAAGCTT TTA CCATTGATAACTCAC-3′) contained a HindIII site (underlined), a stop anticodon (italicized) and anticodons for residues 656–660. The PCR products were cloned in pET20b (Novagen). E. coli Top10 cells were transformed with vectors pET20b-ShuAF1 and pET20b-ShuAF2. The ShuAF2 fragment was removed from the plasmid and introduced into pET20b-ShuAF1 using the BamHI and HindIII restriction sites. E. coli BL21 (DE3) cells were transformed with the resulting construct, which encodes a protein in which the native S. dysenteriae signal peptide is replaced by the native N-terminal pelB signal sequence of E. coli in order to target the outer membrane. The His6 tag in loop L5 allowed the rapid purification of ShuA.
2.2. Expression and purification
Expression was induced with 0.5 mM isopropyl β-d-1-thiogalactopyranoside (IPTG) at 310 K for 3 h. The yield of purified protein was increased by overnight induction. The cells were harvested by centrifugation for 20 min at 10 000g. ShuA was purified after incubation of the membranes with 1%(w/v) sodium N-lauroylsarcosine, leading to solubilization of the cytoplasmic membranes. Intact outer membranes containing ShuA were pelleted by centrifugation at 125 000g for 40 min. The outer membranes were solubilized with 50 mM Tris–HCl pH 8.0, 0.5 M NaCl and 7%(v/v) octyl-polyoxyethylene (octyl-POE; critical micelle concentration 0.23%; Bachem) and subsequently centrifuged at 117 000g for 20 min. The supernatant was applied onto a HisTrap column (Amersham) equilibrated with 50 mM Tris–HCl pH 8.0, 0.5 M NaCl, 20 mM imidazole and 1% octyl-POE. The protein was eluted with a linear gradient of 20–500 mM imidazole in 50 mM Tris–HCl pH 8.0, 0.5 M NaCl and 1% octyl-POE. The fractions containing ShuA were pooled, dialyzed with 50 mM Tris–HCl pH 8.0 and 1% octyl-POE and loaded on a HiTrap Q column (Amersham) equilibrated with 50 mM Tris–HCl pH 8.0 and 1% octyl-POE. ShuA was eluted with a linear gradient of 0–1 M NaCl in 50 mM Tris–HCl pH 8.0 and 1% octyl-POE.
2.3. Haem-binding measurements
The ability of ShuA to bind haem was tested by difference spectroscopy absorbance using a Cary UV–visible spectrophotometer (Varian). Reactions were carried out in 450 µl reaction volumes containing 20 µM ShuA in 10 mM Tris–HCl pH 8.0, 1.4% octyl β-d-glucopyranoside and 20 µM haemin dissolved in 1 mM NaOH. UV–visible absorbance spectra were collected between 300 and 700 nm at room temperature. The step size was 15 nm and the averaging time was 1 s per point.
2.4. Crystallization experiments
The pure fractions containing ShuA were pooled and dialyzed with 10 mM Tris–HCl pH 8.0 and 0.75% octyl-tetraethylene glycol ether (C8E4), 0.75% octyl-pentaethylene glycol ether (C8E5) or 1.4% octyl-β-d-glucopyranoside (β-OG). Initial crystallization experiments were performed using a Cartesian Honeybee system at SBGP (Illkirch, France) and the sitting-drop vapour-diffusion method by mixing 100 nl protein solution at a concentration of 10 mg ml−1 with 100 nl crystallization solution. The MbClass Suite, MbClassII Suite, The Classics Suite and The Classics Lite matrices from Qiagen were tested with the three detergents at 293 K. The reservoir volume was 80 µl. Improvements of the conditions were performed in Linbro plates using the sitting-drop vapour-diffusion method at 293 K by mixing 0.5 µl protein solution (10 mg ml−1) with 0.5 µl crystallization solution. The reservoir volume was 500 µl.
2.5. Cocrystallization with heavy atoms
Solutions of heavy atoms [I2, Pb(NO3)2, EuCl3, TbCl3 and KBr] at 2.8 mM were prepared in 10 mM Tris–HCl pH 8.0 with 1.4% octyl-β-d-glucopyranoside. Pure fractions of ShuA at 10 mg ml−1 were incubated with five or ten molar equivalents of heavy atom at room temperature for 1 h before crystallization.
2.6. Data collection and processing
Diffraction data for ShuA-Pb were collected to 2.3 Å resolution on PROXIMA-1 at SOLEIL using X-rays of wavelength 0.9788 Å and to 3.2 Å resolution (λ = 1.9997 Å) at a high remote energy compared with the M absorption edges of Pb using one crystal at 100 K. Diffraction data for ShuA cocrystallized with TbCl3 and EuCl3 were collected on BM30A (Roth et al., 2002 ▶) at ESRF (λ = 0.9798 Å) and on PROXIMA-1 (λ = 1.7657 Å) at SOLEIL, respectively. All data were processed and scaled using XDS (Kabsch, 1993 ▶).
3. Results and discussion
3.1. High yields of pure and monodisperse apo ShuA
By using an in-house approach from cloning to crystallization of membrane proteins belonging to the TBDT family (Brillet et al., 2009 ▶), we successfully overexpressed and crystallized the haem outer membrane transporter ShuA from S. dysenteriae. As previously observed with other TBDTs (Ferguson et al., 1998 ▶; Brillet et al., 2009 ▶), the insertion of a His6 tag in an extracellular loop of the transporter instead of downstream of the E. coli peptide signal in the pET20b plasmid allowed efficient targeting to the outer membrane. An increase of the overexpression time from 3 to 16 h with 0.5 mM IPTG induction provided the highest yield of ShuA using only a two-step purification (3.6 versus 3 mg l−1). After purification, only one band at approximately 70 kDa (ShuA molecular weight 69.5 kDa) was observed on SDS–PAGE (Fig. 1 ▶). By using gel chromatography, only one peak corresponding to monomeric ShuA was observed. The His6-tagged ShuA was therefore in a monodisperse state before crystallization.
Figure 1.
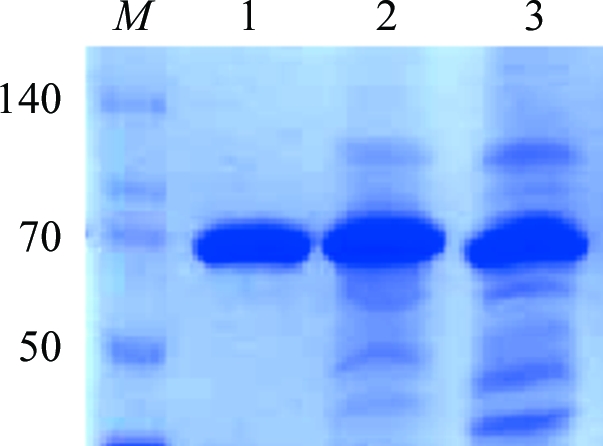
SDS–PAGE of ShuA. Lane M, molecular-weight markers (kDa); lane 1, purified ShuA after anion-exchange chromatography; lane 2, pool after affinity chromatography; lane 3, pool from solubilization of outer membranes.
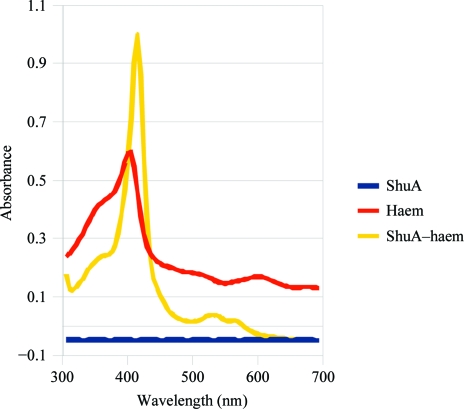
The ShuA–haem complex was characterized by adding haemin to ShuA. The spectral characteristics of haemoproteins were observed, including a Soret peak (maximum at 409 nm) and well resolved α and β bands at approximately 565 and 529 nm (Fig. 2 ▶). These peaks were not observed with ShuA. Similar results were obtained before purification. Contrary to published results (Burkhard & Wilks, 2007 ▶), ShuA does not bind residual haem in E. coli. This difference could result from the expression of ShuA in E. coli as well as membrane isolation and protein purification. Indeed, we quickly solubilized the inner membranes by adding 1% lauroylsarcosine after cell lysis and the solution was centrifuged at 119 000g to pellet the outer membranes. The outer membranes were then subjected to solubilization with 7% octyl-POE. These results showed that ShuA is in its apo form in detergent micelles after purification and binds to haem.
Figure 2.
Spectroscopy experiments with ShuA and ShuA–haem after purification. Experiments were carried out with ShuA before purification and similar results were obtained (data not shown).
3.2. Crystallization and cocrystallization with heavy atoms
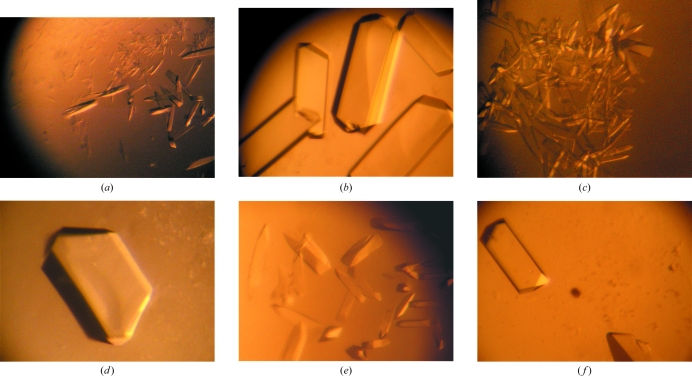
By combining three detergents and four sparse matrices for crystallization, 1152 conditions were tested using only 60 µl protein solution (10 mg ml−1) and several crystallization conditions allowed the production of crystals that diffracted X-rays, but only to low resolution (not beyond 3.5 Å). The conditions mainly used PEG (400, 1K, 2K, 2K MME, 3350 or 4K), salts (NaCl or LiCl) and either HEPES, MES or MOPS buffer in the pH range 6.5–7.5. No crystals obtained with PEG 400 diffracted X-rays. Improvement of the crystallization conditions was achieved using heavy atoms, as had previously been observed with ribosome (Yonath, 1992 ▶). Cocrystallization experiments with Pb(NO3)2, EuCl3, TbCl3, KBr or I2 were undertaken because their anomalous signals (Pb, I, Tb and Eu) can be detected using a copper rotating-anode generator. A SAD data set could be then collected. These ions are therefore of interest for testing at the laboratory before a three-wavelength MAD experiment. Cocrystallization with positively charged heavy atoms strongly affected the shape and size of the ShuA crystals (Fig. 3 ▶). The number of crystals per drop also decreased and the crystals often diffracted to beyond 3.5 Å resolution. The crystallization conditions are given in Fig. 3 ▶.
Figure 3.
Crystals of ShuA (a, c, e), ShuA-Pb (b), ShuA-Tb (d) and ShuA-Eu (f). All crystals were obtained using either 13–15% PEG 1K (a, b), 11–14% PEG 1.5K (c, d) or 8–12% PEG 3350 (e, f) with 0.1 M MES pH 6.5 and 0.1 M NaCl. The protein concentration was 10 mg ml−1 in 10 mM Tris–HCl pH 8.0 with 1.4% octyl-β-d-glucopyranoside. The heavy-atom concentration in the protein solution was 1.4 mM.
3.3. Data collection and processing
Prior to data collection, the ShuA, ShuA-Pb, ShuA-Eu or ShuA-Tb crystals were frozen in liquid nitrogen after a brief soak in the initial crystallization solution with 15%(v/v) glycerol. A data set was collected to 2.3 Å resolution on PROXIMA-1 at SOLEIL using one crystal of ShuA-Pb and data were processed at 2.6 Å resolution. Systematic absences (2n + 1) were observed for the h00, 0k0 and 00l reflections resulting from the presence of 21 axes. The crystals belonged to space group P212121, with unit-cell parameters a = 78.10, b = 114.21, c = 117.09 Å. The addition of Pb2+ or other positively charged ions (Eu3+ or Tb3+) to the protein solution before crystallization improved the resolution limits. A second data set was collected at 3.2 Å resolution using X-rays with wavelength 1.9997 Å on PROXIMA-1 (12 < Pb f′′ < 13 electrons) and the same crystal of ShuA-Pb. The data statistics are summarized in Table 1 ▶. The crystal contained approximately 65% solvent and one molecule was present in the asymmetric unit. It belonged to the type II crystals of membrane proteins (Michel, 1983 ▶). ShuA and ShuA derivatives (Pb2+, Eu3+ and Tb3+) crystallized in the same space group with similar unit-cell parameters. Analyses of the electron-density maps calculated for ShuA-Pb, ShuA-Tb and ShuA-Eu should provide further details about the binding sites and the effects of the heavy atoms on crystallization.
Table 1. X-ray data statistics for ShuA-Pb.
The unit-cell parameters are a = 78.10, b = 114.21, c = 117.09 Å and the space group is P212121. Values in parentheses are for the highest resolution shell.
| Wavelength (Å) | 0.9788 | 1.9997 |
| Resolution limits (Å) | 19.8–2.6 (2.7–2.6) | 33.2–3.2 (3.3–3.2) |
| Total reflections | 232991 (25051) | 135194 |
| Unique reflections | 32798 (3433) | 32661 |
| Completeness (%) | 99.7 (99.9) | 99.5 (98.5) |
| Rmerge (%)† | 9.0 (44.8) | 6.1 (27.3) |
| I/σ(I) | 16.27 (4.68) | 16.65 (5.18) |
R
merge = 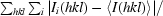
 , where Ii(hkl) is the intensity of the measured reflection and 〈I(hkl)〉 is the mean intensity of this reflection.
, where Ii(hkl) is the intensity of the measured reflection and 〈I(hkl)〉 is the mean intensity of this reflection.
By using an in-house approach for membrane protein crystallization (Brillet et al., 2009 ▶), we overexpressed, crystallized and collected diffraction data for ShuA, the haem TBDT from S. dysenteriae. Improvement of the crystal quality by adding heavy atoms with strong anomalous signals at the Cu wavelength showed that these ions and/or other lanthanides (for example) could be used as additives for crystallization. If the SeMet-substituted protein cannot be overexpressed or if the protein does not contain methionine residues, SAD phasing in the laboratory and a MAD experiment at the synchrotron can be performed with such anomalous scatters if they are bound to the protein.
Acknowledgments
We thank Dr Shelley M. Payne of the Section for Molecular Genetics and Microbiology, University of Texas at Austin for providing us with the DNA encoding ShuA. We thank the BL14 staff at BESSY and the people of the MX group and the BM30A beamline at ESRF for their kind assistance during data collection. This work was supported by the Ministère de l’Enseignement Supérieur et de la Recherche and the Centre National de la Recherche Scientifique.
References
- Brillet, K., Meksem, A., Lauber, E., Reimmann, C. & Cobessi, D. (2009). Acta Cryst. D65, 326–331. [DOI] [PubMed] [Google Scholar]
- Burkhard, K. A. & Wilks, A. (2007). J. Biol. Chem.228, 15126–15136. [DOI] [PubMed]
- Cescau, S., Cwerman, H., Letoffe, S., Delepelaire, P., Wandersman, C. & Biville, F. (2007). Biometals, 20, 603–613. [DOI] [PubMed]
- Ferguson, A. D., Breed, J., Diederichs, K., Welte, W. & Coulton, J. W. (1998). Protein Sci.7, 1636–1638. [DOI] [PMC free article] [PubMed]
- Ferguson, A. D. & Deisenhofer, J. (2002). Biochim. Biophys. Acta, 165, 318–332. [DOI] [PubMed]
- Kabsch, W. (1993). J. Appl. Cryst.26, 795–800.
- Krewulak, K. D. & Vogel, H. J. (2008). Biochim. Biophys. Acta, 1778, 1781–1804. [DOI] [PubMed]
- Michel, H. (1983). Trends Biochem. Sci.8, 56–59.
- Mills, M. & Payne, S. M. (1997). Infect. Immun.65, 5358–5363. [DOI] [PMC free article] [PubMed]
- Neilands, J. B. (1995). J. Biol. Chem.270, 26723–26726. [DOI] [PubMed]
- Paulley, J. T., Anderson, E. S. & Roop, R. M. (2007). Infect. Immun.75, 5248–5254. [DOI] [PMC free article] [PubMed]
- Roth, M., Carpentier, P., Kaïkati, O., Joly, J., Charrault, P., Pirocchi, M., Kahn, R., Fanchon, E., Jacquamet, L., Borel, F., Bertoni, A., Israel-Gouy, P. & Ferrer, J.-L. (2002). Acta Cryst. D58, 805–814. [DOI] [PubMed]
- Stintzi, A., Barnes, C., Xu, J. & Raymond, K. N. (2000). Proc. Natl Acad. Sci. USA, 97, 10691–10696. [DOI] [PMC free article] [PubMed]
- Yonath, A. (1992). Annu. Rev. Biophys. Biomol. Struct.21, 77–93. [DOI] [PubMed]