Abstract
We explored the molecular mechanisms of morphological transformations of vertebrate paired fin/limb evolution by comparative gene expression profiling and functional analyses. In this study, we focused on the temporal differences of the onset of Sonic hedgehog (Shh) expression in paired appendages among different vertebrates. In limb buds of chick and mouse, Shh expression is activated as soon as there is a morphological bud, concomitant with Hoxd10 expression. In dogfish (Scyliorhinus canicula), however, we found that Shh was transcribed late in fin development, concomitant with Hoxd13 expression. We utilized zebrafish as a model to determine whether quantitative changes in hox expression alter the timing of shh expression in pectoral fins of zebrafish embryos. We found that the temporal shift of Shh activity altered the size of endoskeletal elements in paired fins of zebrafish and dogfish. Thus, a threshold level of hox expression determines the onset of shh expression, and the subsequent heterochronic shift of Shh activity can affect the size of the fin endoskeleton. This process may have facilitated major morphological changes in paired appendages during vertebrate limb evolution.
Introduction
There has been considerable debate regarding the fundamental mechanisms that direct morphological transformations from fins into limbs with respect to the expression patterns of 5′-located Hox genes and subsequent Shh expression [1], [2], [3]. It is generally accepted, however, that, the enlargement of the fin endoskeleton along the proximal-distal axis within the lineage of basal sarcopterygians (lobe-finned fishes) results from changes in the heterochronic folding of the apical fin fold [4]; other possibilities have scarcely been discussed. Here we have investigated the genetic basis of morphological transitions of the vertebrate fin endoskeleton primarily via comparative gene expression profiling and functional analyses, focusing especially on the temporal onset of Shh expression. Because two paired appendages are unique to gnathostomes—and cartilaginous fish occupy the earliest branch of the gnathostome lineage—the study of the cartilaginous dogfish may provide insight into how animals have acquired morphologically diverse paired appendages. Although the developmental mechanisms of such morphological changes are still under debate, the evolutionary acquisition of Shh function in growing paired appendages might have been a crucial step in implementing morphological innovations of paired appendages.
Patterning along the anterior-posterior axis of the limb is controlled by signalling from the posterior margin of the limb bud, the polarizing region discovered by Saunders and Gasseling [5]. Grafted tissue from the polarizing region of a chick limb bud to the anterior margin of another chick limb bud resulted in remarkable mirror-image symmetry of digits. Several subsequent studies showed that this polarizing activity involves a dose-dependent response because the identity of the additional digits that form depends on the number of grafted cells from the polarizing region [6], [7]. Sonic hedgehog (Shh), which encodes a secreted factor, was later found to be expressed precisely in those cells identified as the polarizing regions in the limb buds of chick and mouse [8], [9] and also in zebrafish fin buds [10]. Application of Shh-expressing cells or an Shh-soaked bead into the anterior margin of chick limb buds induced the same type of dose-dependent mirror-image digit patterns as a graft tissue from the polarizing region [8], [11]. More recently, it was shown that the length of time that cells are exposed to Shh, in addition to the Shh dose, is crucial for the patterning of the digital plate [12], [13]. Subsequent experiments demonstrated that the longer the limb bud cells are exposed to Shh, the more posterior digits are formed [13]. Furthermore, recent studies demonstrated that Shh can regulate not only digit specificity but also cell proliferation in limb buds of chick and mouse embryos [14], [15]. Similarly, a requirement for shh acitivity in cell proliferation in the zebrafish pectoral fin bud has also been suggested [16]. These results raise the possibility that the duration of exposure to Shh activity may have been critical for the morphological evolution of paired appendages.
To investigate the possibility that the duration of exposure to Shh activity may have been critical for the morphological evolution of paired appendages, we analyzed fin development in embryos of the cartilaginous dogfish Scyliorhinus canicula. In limb buds of chick and mouse, Shh expression is activated as soon as there is a morphological bud, whereas in S. canicula fin buds, consistent with reported data in other cartilaginous fishes [17], Shh is transcribed late in fin development. Several molecular triggers that activate Shh expression have been proposed, including Hand2 and Fibroblast growth factor (Fgf) [18], [19]. In pectoral fins of S. canicula, Hand2 transcripts localize posteriorly at a much earlier stage than Shh transcripts, and it is therefore unlikely that Hand2 correlates directly with the late onset of Shh transcription [20]. In vertebrate limb buds, Fgfs are secreted from the apical ectodermal ridge that rims the distal edge of the buds, and these Fgfs play pivotal roles in limb bud initiation and outgrowth, at least in part by inducing and maintaining the expression of Shh in the underlying mesenchyme [19], [21], [22], [23], [24]. Hoxa and Hoxd have also been demonstrated to drive Shh expression in mouse limb buds [2], [3], [25]. Furthermore, recent experiments have shown that Hox proteins bind to a conserved regulatory region of Shh, thereby promoting Shh expression within developing mouse limb buds [26]. In our current study, we show that a threshold level of hox expression is essential for the onset of shh expression and that the subsequent heterochronic shift of Shh activity leads to changes in the size of pectoral fins. These results imply that a quantitative change in hox expression could have involved a heterochronic shift of shh expression and subsequent morphological changes of endoskeleton during limb evolution.
Materials and Methods
Animals
S. canicula eggs were incubated at 12∼16°C in sea water and staged according to Ballard et al. (1993). The gross duration of incubation described in Ballard et al. (1993) was as follows: stage 27 (42–46 days), stage 29 (49–53 days), stage 32 (75–125 days). Because duration of stage 32 is long, we subdivided stage 32 into “early stage 32” (75–100 days) and “late stage 32” (101–125 days). Wild-type (TL strain and AB/Tübingen strain) zebrafish (Danio rerio) were maintained at 28.5°C and staged using standard morphological criteria [27].
Identification of S. canicula gene homologs
We identified fragments of S. canicula (Sc) Fgf8 (296 bp), Meis1 (357 bp), Hoxa11 (357 bp), Hoxa13 (389 bp), Hoxd11 (534 bp), Hoxd13 (296 bp), Pbx2 (653 bp), Ptc2 (1157 bp) and GAPDH (230 bp) from cDNA pools prepared from stage 24–30 embryos using degenerate primers. The degenerate primers were designed to anneal to coding regions containing the following amino acid sequences: ScFgf8, TYQLYSRT and VHFMKRL; ScMeis1, CDNFCHR and GIFPKVA; ScHoxa11, QVQPVRE and AATSSS; ScHoxa13, AYTSSEV and PMESYQP; ScHoxd11, CQMTFPYS and PYTKYQIR; ScHoxd13, PVEKYMDV and IWFQNRRV; ScPbx2, QQIMTIT and PYPSEEA; ScPtc2, IHAFSTT and QFKYFSFYNF; ScGAPDH, ASCTTN and VIPELN. S. canicula ScHoxd10 (785 bp) and ScHoxd12 cDNAs (316 bp) were amplified by PCR using the following primers which hybridized to the indicated published sequence: ScHoxd10, GenBank accession number DQ659105, 5′-GGGAACATACGGAATGCAGACC-3′ and 5′-GTAAGAGCGTGAATCTGACCG-3′; ScHoxd12, GenBank accession number DQ659106, 5′-CCCTTCTATTTCGCCAACCTG-3′ and 5′-CCCAAGTGATACCAGCATCC-3′. The nucleotide sequences of the ScFgf8, ScHoxd13, ScMeis1, ScHoxd11, ScHoxa11, ScHoxa13, ScPbx2, ScPtc2 and ScGAPDH cDNAs were deposited in the GenBank database under the accession numbers: DQ647321–DQ647323, DQ854846, EU005549–EU005551, EU814484 and EU826015, respectively.
Whole-mount in situ hybridization and immunohistochemistry
S. canicula embryos were removed from their egg casings and dissected from the yolk mass. Whole-mount in situ hybridization of S. canicula and immunostaining of S. canicula embryos were carried out as described [20]. Whole-mount in situ hybridization of zebrafish was performed as described [28]. Probes for zebrafish hoxd10a, hoxd11a and hoxd13a were amplified by reverse transcription-polymerase chain reaction (RT-PCR) using primers derived from published sequences (www.ensembl.org). For whole-mount immunostaining, embryos were prepared as described [29]. The monoclonal antibody against human Fgf4 (R & D Systems) was used at a 1∶300 dilution.
Microinjection
For mRNA injection, the full-length cDNAs encoding hoxa13a, hoxd10a, hoxd13a, hoxd4 and pbx2 were individually cloned into the pCS2+ vector and the corresponding mRNAs were synthesized using the MEGAscript kit (Ambion). The mRNAs were dissolved in endotoxin-free H2O to a final concentration of 20 mg/ml. Morpholino antisense oligonucleotides (MOs) were obtained from Gene Tools, Inc. The following hoxd10a and hoxd13a MOs targeted the boundary between exon 1 and intron 1 of each respective gene (Gene Tools, Inc.): MO-hoxd10a, CCGTTTATTGTACCCACCTTTGCCT; MO-hoxd13a, CAGAGCTGAGGTCTTACCTGTTAAT. The pbx2 MO was used as described [30]. The standard control MO obtained from Gene Tools, Inc. was used as an injection control. MOs were dissolved in sterile H2O at concentrations of 1, 2.5 or 5 mg/ml and phenol red was added to the solution. Approximately 1 nl of mRNA or MO was injected at the one-cell stage using a microinjector (IM30, Narishige).
To test the efficiency of the hoxd10a-MO and the hoxd13a-MO, RT-PCR was performed using total RNA from 30 embryos at 24 hpf to detect spliced and unspliced hoxd10a or hoxd13a mRNAs. The following PCR primers for hoxd10a and hoxd13a were used for amplification: hoxd10a, 5′-TGTCCACCTGCACATTTTCAC-3′ and 5′-CTTGTCTGTCAGTCAGGTTGACGC-3′; hoxd13a, 5′-GAGATCTTAGACATGAGACTTG-3′ and 5′-CCTCTTTGAATTCGAGATTCTC-3′. Amplification of eif4a transcripts was used as a control [31].
Semi-quantitative and quantitative expression analysis
Lateral plate mesoderm overlying the yolk of zebrafish embryos and pectoral fin buds of dogfish embryos were isolated by dissection. Total RNA was extracted from dissected embryos using the RNeasy Mini kit (Qiagen). To remove genomic DNA, each RNA sample was treated with RNase-free DNase (Qiagen). The RNA was used as a template for synthesizing cDNA using AMV Reverse Transcriptase (Promega). The following PCR primers for ScFgf8 were used for amplification: 5′-AGATTAACGCAAAGGCGGAGG-3′ and 5′-GAATCAATGCTACTGCTGAAG-3′. For semi-quantitative RT-PCR, spliced, functional hoxd10a,hoxd11a and ScShh transcripts were amplified with the following primers: hoxd10a, 5′-CCAAAGTCAGCACGCTGGAG-3′ and 5′-CTCCCGAGTCAGATACATGTTG-3′; hoxd11a, 5′-ACACCGTGGAGGAGGAATCC-3′ and 5′-CGTTCAAGTTCTCGGATCTGG-3′; ScShh, 5′-CTGACAGGCTGATGACACAG-3′ and 5′-ATCCCGTACTTGGTTCGGTC-3′. To determine relative transcript levels of functional hoxd10a,hoxd11a, and ScShh RT-PCR products were subjected to agarose gel electrophoresis, soaked in a 1 µg/ml ethidium bromide solution, and the intensity of each band was measured using the ImageJ program (National Institute of Health, Bethesda, MD). For quantitative real-time RT-PCR, we used the 7300 real-time PCR System (Applied Biosystems) with SYBR Green I. hoxd11a, shh, ScHoxd10, ScHoxd11, ScHoxd12 and ScHoxd13 transcripts were amplified with the following primers: hoxd11a, 5′- CCGTTTCAACCTGCGATGAAG -3′ and 5′- CGTTCAAGTTCTCGGATCTGG -3′; shh, 5′-TTGACTGGGTCTATTACGAGTCC-3′ and 5′-GGTTCAGGTCCTTCACGGCCTTC -3′; ScHoxd10, 5′- GAACTATCGGACAATGAGAC -3′ and 5′- CGGTCAGATTCACGCTCTTAC -3′; ScHoxd11, 5′- TCGGACACCTCTAACTATGAAC -3′ and 5′- ACACTGTTACCGGAGGACTC -3′; ScHoxd12, 5′- CCCTTCTATTTCGCCAACCTG -3′ and 5′- TGATGGAGACTGAGTTGCTG -3′; ScHoxd13, 5′- ACTGACGAGGTGTCATCCAG -3′ and 5′- TGCATCGCAGGTTAGTGGATAG -3′.
The relative expression level of each gene was normalized to gapdh expression [32] for zebrafish and ScGAPDH expression for dogfish embryos. Each standard deviation was calculated using data from three independent experiments.
Cyclopamine and SAG treatment
To investigate the effect of hedgehog (hh) signaling on pectoral fin buds, zebrafish embryos were treated from 23 hpf to 27 or 57 hpf with either 0.6% (v/v) ethanol in fish water (vehicle) [28] or with 60 µM cyclopamine (Biomol), a hh signaling antagonist, dissolved in vehicle. Incubation with cyclopaminewas terminated by washing in fish water, and embryos were incubated until fixation. To examine the effect of hh signaling on adaxial cells, zebrafish embryos were treated from the 1-cell-stage to the 8-somite-stage with either 1.0% (v/v) ethanol in fish water (vehicle) [28], 100 µM SAG (Alexis), a hh signaling agonist, or 100 µM cyclopamine in vehicle.
Dogfish embryos were treated for 4 days from stage 28 with cyclopamine or 6 days from stage 30 with SAG. Briefly, 50 µl of 10 mM cyclopamine dissolved in ethanol or 25 µl of 100 µM SAG dissolved in ethanol was injected into the dogfish egg case, which then was reared in seawater. For SAG treatment, 25 µl of 100 µM SAG was added 3 days after the first day of treatment. Control embryos were reared in seawater. Incubation with cyclopamine or with SAG was terminated by washing in seawater several times, and embryos were reared in seawater until fixation.
Cartilage staining
Cartilage staining was conducted as described [33].
Results
Shh is transcribed late in S. canicula development, concomitant with Hoxd13 expression
The evolutionary acquisition of Shh function into growing paired appendages might have been crucial in implementing the morphological evolution of tetrapod appendages. We previously reported that Shh expression could not be detected in the fin buds of dogfish (Scyliorhinus canicula) embryos at stage 27 [20] and further studies have confirmed this finding (Fig. 1A). In addition, however, when we examined fin buds at much later stage 29, we detected posterior Shh expression (Fig. 1A). By early stage 32, Shh expression became downregulated in fin buds (Fig. 1A), as confirmed by RT-PCR analysis (Fig. S1)[20]. In contrast, Shh expression in chick and mouse is activated as soon as there is a morphological bud and persists at least until the distal region that will give rise to digits is produced [8]. This suggests that temporal shifts in the Shh expression during vertebrate limb evolution might have led to major morphological innovations and diversification in paired appendages. To explore this possibility further, we investigated several genetic components that may have contributed to acquisition of Shh expression in fins at this late stage of development in dogfish. We first examined whether Fgf signalling in S. canicula fins is reduced and/or delayed, leading to a delay in Shh expression in fin buds. Although the distal edge of S. canicula fin buds has an ectodermal structure called the apical fin fold that is similar to the apical ridge of limb buds of higher vertebrates, it is not known whether the apical fin fold produces Fgf. It is possible that Fgf is not produced at a time that would influence Shh expression. Therefore we isolated cDNA fragments of Fgf8 from S. canicula embryos and examined their expression patterns at stages 27–32 (Fig. S1B–D). In situ hybridization experiments showed that Fgf8 was expressed in the developing gill filaments and nasal pits of stage 27 S. canicula embryos (Fig. S1B). In contrast, Fgf8 transcripts could not be detected in the apical fin folds at any stage examined (Fig. S1C–E). We also investigated production of Fgfs using an antibody against Fgf4. We found that anti-Fgf4 antibody-positive cells were distributed in the apical ectodermal fold at stage 27 (Fig. S1F). Wnt signaling induces Fgf expression via a β-catenin-dependent pathway in limb bud–forming regions in vertebrates. To test the probe efficacy in the apical fin fold of S. canicula fins, we isolated β-catenin cDNA fragments and examined their expression pattern. In S. canicula fins at stages 27 (not shown) to 32 (Fig. S1G), abundant β-catenin transcripts were observed, including in the apical fin fold (arrows in Fig. S1G), demonstrating probe efficacy. These results indicated that signaling by Fgfs occurs at early fin bud stages in S. canicula and may be involved in fin patterning and outgrowth. We therefore concluded that the late onset of Shh transcription in fin buds is not due to a delay in Fgf expression during the early bud stages.
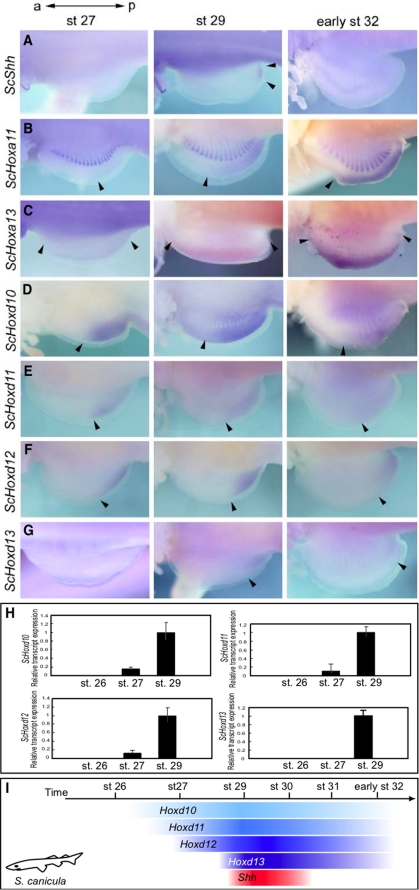
Figure 1. Shh expression commences late in S. canicula (Sc) fin development, concomitant with Hoxd13 expression.
(A–G) Pectoral fin buds. Anterior is to the left. (A) ScShh expression at stages 27, 29 and early stage 32. Transcripts were present in the posterior region (arrowheads) at stage 29 but absent at stages 27 and early stage 32. (B, C) Expression of ScHoxa11 (B) and ScHoxa13 (C). ScHoxa11 transcripts were first detected in the posterior region and in the muscle buds. By early stage 32, transcripts were restricted to the posterior-distal region. ScHoxa13 transcripts were restricted to the distal part of the fin buds throughout fin development. Arrowheads indicate limits of ScHoxa expression. (D–G) Expression of ScHoxd10 (D), ScHoxd11 (E), ScHoxd12 (F) and ScHoxd13 (G). The ScHoxd genes were expressed collinearly at early stages. ScHoxd10–d12 transcripts were apparent at stage 27, whereas ScHoxd13 transcripts were first observed in the posterior mesenchyme at stage 29. Arrowheads indicate the anterior limits of ScHoxd expression. (H) Quantitative PCR analysis to determine the expression levels of ScHoxd10–13 in the pectoral fins of stage 26, 27 and 29 dogfish embryos. Relative expression was normalized against ScGAPDH transcripts. Note that levels of ScHoxd10–13 transcript expression increased at stage 29. Expression of ScHoxd10–d13 in stage 26 pectoral fins, or expression of ScHoxd13 in stage 27 pectoral fins, was not detectable. (I) Schematic representation of temporal Hoxd expression and Shh expression during pectoral fin development in S. canicula. Shh was expressed concomitantly with Hoxd13.
The Hox genes have recently been shown to regulate Shh transcription in developing mouse limb buds. In higher vertebrates, ectopic Hox expression leads to Shh transcription, whereas functional ablation of Hox genes leads to distal limb truncations caused by the absence of Shh expression [2]. To investigate whether the late onset of Shh transcription in fin buds of S. canicula is regulated by Hox genes, we isolated cDNA fragments of the 5′-located Hoxa and Hoxd genes, such as Hoxa11, Hoxa13, Hoxd10, Hoxd11, Hoxd12 and Hoxd13 from S. canicula and examined their expression patterns. Very weak hybridization signal was seen for Hoxa11 in the posterior fin buds and muscle buds at stage 27, but this signal intensified in later stages (Fig. 1B). Hoxa13 expression appeared at stage 27 in the distal region and persisted in the same region at least until early stage 32 (Fig. 1C). Thus, expression of 5′ -located Hoxa genes in the developing pectoral fins in S. canicula was greater at stage 29 than at stage 27 and remained nested and overlapping throughout development in a manner remarkably similar to that seen in zebrafish [1] and Polydon spathula [34].
Collinear expression of Hoxd genes was also observed in the pectoral fins, in accordance with previous results [35]. Hybridization signals for Hoxd10–12 were seen in the posterior region of the pectoral fins in a nested manner at stage 27 (Fig. 1D–F), whereas no transcripts of the 5′-most Hoxd gene, Hoxd13, were detected in pectoral fins of stage 27 embryos (Fig. 1G). By stage 29, when Shh expression is turned on, Hoxd10–12 expression had increased (Fig. 1D–F), and Hoxd13 expression appeared in the posterior part of the pectoral fin buds (Fig. 1G). At early stage 32, Hoxd10 expression persisted in the posterior fins, but expression of Hoxd11–13 had decreased (Fig. 1D–G). Thus, Shh expression was transcribed at stage 29 concomitantly with Hoxd13 expression in pectoral fins of S. canicula embryos (Fig. 1I). To quantify the expression levels of S. canicula Hoxd10–13 (ScHoxd10–13) in pectoral fin buds of embryos, we performed quantitative real-time PCR using total RNA from pectoral fin buds at stages 26, 27 and 29 (Fig. 1H). ScHoxd10–13 mRNA levels in pectoral fin buds had dramatically increased by stage 29 (Fig. 1H). These results suggested that the temporal expression of Hox in the pectoral fins may correlate with the late onset of Shh transcription in S. canicula embryos.
The level of hox transcripts is critical for the onset of shh expression in pectoral fin primordia of zebrafish embryos
In the dogfish S. canicula pectoral fins, Shh, which is transcribed at a late stage in fin development, was expressed at the same time as Hoxd13 (Fig. 1I). In contrast, shh expression in zebrafish occurred at 24 hours post-fertilization (hpf) and was concomitant with hoxd10a expression in pectoral fin primordia (Figs. 2A and C, Fig. S2). To address whether expression of the 5′-hox genes could shift the onset of shh transcription in pectoral fin primordia, we manipulated the expression levels of specific hox transcripts in the zebrafish model system (Figs. 2 and 3).
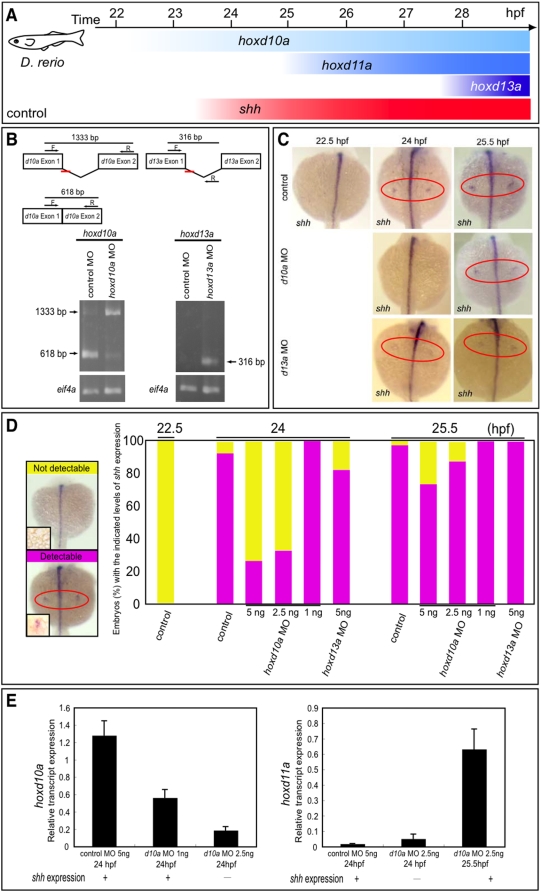
Figure 2. Timing of shh expression in zebrafish embryo fin primordia depends on hox transcript accumulation.
(A) Schematic representation of temporal hox and shh expression in the pectoral fin primordia of zebrafish embryos. shh was expressed at 24 hpf concomitantly with hoxd10a expression. (B) RT-PCR analysis to determine the efficiency of the hoxd10a or hoxd13a splice-blocking morpholino (MO). In the schematics, arrows represent forward (F) and reverse (R) primers, and the short red bars represent the hoxd10a MO and hoxd13a MO. Lower panel, analysis of RT-PCR products by agarose gel electrophoresis. Products of 618 bp and 1333 bp represent spliced and unspliced hoxd10a mRNA, respectively. The 316-bp RT-PCR product represents spliced hoxd13a mRNA. Amplification of eif4a cDNA was used as a control. (C) Whole-mount in situ hybridization to detect shh expression in the pectoral fin primordia of D. rerio embryos injected with 5 ng control MO (top panels), 5 ng hoxd10a MO (middle panels) or 5 ng hoxd13a MO (bottom panels) at the indicated hpf. Red ovals highlight the pectoral fin primordia. Note that shh expression was first observed at 24 hpf in the fin primordia of embryos injected with control (top) or hoxd13a MO (bottom), whereas shh transcripts became detectable at 25.5 hpf in the primordia of most embryos injected with hoxd10a MO (middle). (D) Percentages of embryos with detectable or undetectable levels of shh expression observed at 22.5, 24, and 25.5 hpf following injection of control MO, hoxd10a MO or hoxd13a MO (see also Figure S4). A representative image depicting the detectable or undetectable levels of shh expression in the pectoral fin primordia is shown at the left. Insets show high magnification views of pectoral fin primordia. (E) Semi-quantitative RT-PCR analysis to determine the expression levels of 5′ hoxd when shh is transcribed in pectoral fin buds. The relative levels of hoxd10a and hoxd11a transcripts in the lateral plate mesoderm of morphants were quantified. Relative expression was normalized against gapdh transcripts.
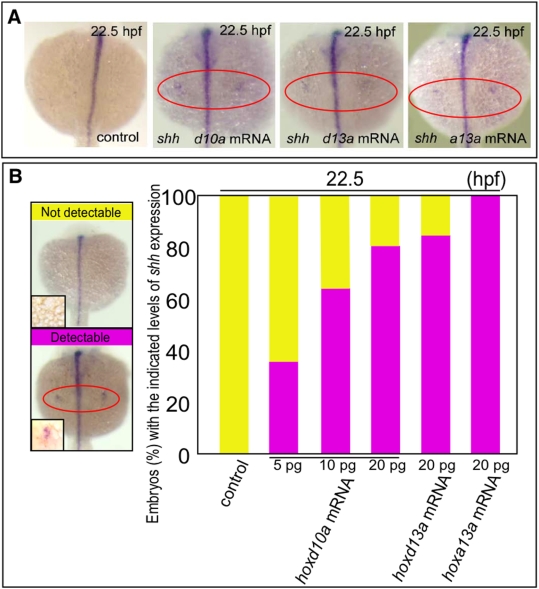
Figure 3. hox transcript accumulation is critical for the onset of shh expression in fin development.
(A) Expression of shh in pectoral fin primordia of D. rerio embryos injected with 5 ng control MO, 20 pg hoxd10a mRNA, 20 pg hoxd13a mRNA or 20 pg hoxa13a mRNA at 22.5 hpf. Red ovals highlight the pectoral fin primordia. Note that transcripts of shh became detectable at 22.5 hpf in the fin primordia of embryos injected with hoxd10a, hoxd13a or hoxa13a mRNA. (B) The percentage of embryos with the indicated level of shh expression at 22.5 hpf following injection of control MO, hoxd10a mRNA, hoxd13a mRNA or hoxa13a mRNA is shown in the bottom panel (see also Figure S4). A representative image depicting the detectable or undetectable levels of shh expression in the pectoral fin primordia is shown at the left.
We used an antisense morpholino oligonucleotide (MO) to change the levels of hoxd10a or hoxd13a transcripts. The MOs were designed to inhibit splicing of hoxd10a or hoxd13a pre-mRNA, leading to the knockdown of hoxd10a or hoxd13a function. Unspliced hoxd10a transcripts were detectable by RT-PCR in embryos injected with 7.5 ng of the hoxd10a MO (1333-bp band in Fig. 2B, lower panel), whereas in embryos injected with the control MO, spliced hoxd10a mRNAs were detected (618-bp band in Fig. 2B, lower panel). We also detected unspliced hoxd13a transcripts in embryos injected with 7.5 ng of the hoxd13a MO, (316-bp band in Fig. 2B, lower panel), whereas no band was detected in embryos injected with 5 ng of the control MO (Fig. 2B, lower panel). These results demonstrated that the MOs targeting hoxd10a and hoxd13a efficiently blocked production of the mature hoxd10a and hoxd13a spliced transcripts.
We then examined the pectoral fins of hoxd10a or hoxd13a zebrafish morphants with those of control morphants at 24 and 25.5 hpf. Expression of shh was first observed in pectoral fin primordia of 24 hpf embryos injected with 5 ng control MO (91.2% of morphants, n = 34, Figs. 2C and D, Fig. S4). When 5 ng of hoxd10a MO was used, however, shh expression was initiated in only 28.1% of 24 hpf embryos (n = 32); by 25.5 hpf, shh was expressedin 72.7% of morphants (n = 33, Figs. 2C and D, Fig. S4). This delay in the onset of shh expression was also observed in 70.0% of embryos injected with 2.5 ng of hoxd10a MO (n = 30, Fig. 2D, Fig. S4). However, injection of a lower concentration of hoxd10a MO (1 ng) did not cause a delay in onset of shh expression in any morphants (n = 30, Fig. 2D, Fig. S4). In zebrafish, hoxd13a expression appeared in pectoral fin primordia at a much later stage (28 hpf, Fig. S2) than shh (24 hpf, Figs 2C). When we injected 5 ng hoxd13a MO into eggs, shh expression was observed in pectoral fin primordia of 24 hpf morphants (81.8%, n = 22, Figs. 2C and D, Fig. S4), similar to that for embryos injected with 5 ng control MO at 24 hpf (91.4%, n = 34, Figs. 2C and D, Fig. S4). Semi-quantitative RT-PCR showed that injection of increasing amounts of hoxd10a MO efficiently reduced the amount of spliced, functional hoxd10a transcripts in a dose-dependent manner from the lateral plate mesoderm of zebrafish morphants at 24 hpf (Fig. 2E). Transcription of shh also was first observed at 24 hpf in the pectoral fin primordia of embryos injected with 1 ng of hoxd10a MO (Fig. 2D), although the amount of spliced hoxd10a transcripts was reduced to 50% of that of control embryos (Fig. 2E). In contrast, shh expression was not observed in the pectoral fin primordia of zebrafish embryos injected with 2.5 ng hoxd10a MO (Fig. 2D), in which the amount of spliced hoxd10a transcripts was reduced to 15% of that of control embryos (Fig. 2E). Transcripts of functional hoxd11a were barely detectable in pectoral fin primordia of zebrafish embryos injected with either control MO or 2.5 ng hoxd10a MO. Expression of shh could be detected by in situ hybridization (Fig. 2C) by 25.5 hpf, when hoxd11a expression was detected in hoxd10a morphants (Fig. 2E), although the amount of functional hoxd10a transcripts was still effectively reduced. Results from the real-time quantitative RT-PCR analyses confirmed these observations (Fig. S3).
Because the onset of shh expression in hoxd10a morphants coincided with the onset of hoxd11a expression (Fig. 2C and 4), it is possible that shh is transcribed only when a certain threshold level of accumulated hox is present in zebrafish pectoral fin primordia. To test this hypothesis, we injected hoxd10a mRNA or hoxd13a mRNA into embryos and investigated whether excess amounts of hoxd mRNA could accelerate the timing of onset of shh expression in pectoral fin primordia. Although control embryos did not express shh in pectoral fin primordia at 22.5 hpf (0%, n = 32, Fig. 3A, Fig. S4), 88% of embryos injected with 20 pg hoxd10a mRNA expressed shh in pectoral fin primordia at 22.5 hpf (n = 25, Fig. 3, Fig. S4). In embryos injected with hoxd10a mRNA, the onset of shh expression was accelerated in a dose-dependent manner (Fig. 3B, Fig. S4). These observations were confirmed by real-time quantitative RT-PCR analyses (Fig. S3D). Likewise, shh transcripts appeared at 22.5 hpf in 82.6% of embryos injected with 20 pg hoxd13a mRNA (n = 23, Fig. 3, Fig. S4). Thus, expression levels of hoxd are crucial for the timing of shh expression in zebrafish fin primordia. In mouse limb buds, Hoxa genes, as well as Hoxd genes, are involved in regulation of Shh expression [3]. We therefore investigated whether the onset of shh expression in fin primordia could also be triggered by a threshold level of hoxa. At 22.5 hpf, shh expression was seen in pectoral fin primordia in 100% of zebrafish embryos injected with 20 pg hoxa13a mRNA (n = 27, Fig. 3, Fig. S4). Thus, expressing a threshold level of hoxa could also trigger shh expression in pectoral fin primordia (Fig. 4). Our results indicate that specific threshold levels of hox gene products likely trigger the heterochronic shift of shh expression in pectoral fin primordia.
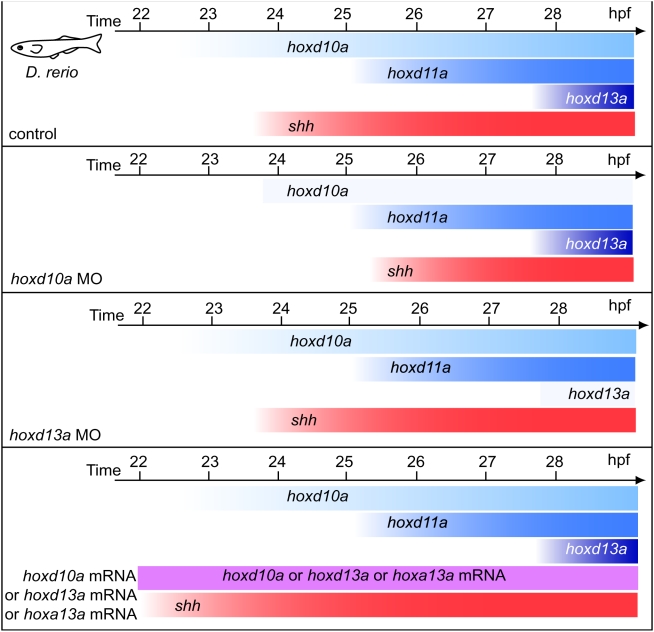
Figure 4. Schematic representation of temporal hox and shh expression in pectoral fin primordia of zebrafish embryos.
Expression of shh was observed at 24 hpf and was concomitant with hoxd10a expression. The onset of shh expression in hoxd10a morphants was concomitant with the onset of hoxd11a expression, whereas shh expression was not delayed in hoxd13a morphants. In embryos injected with hoxd10a, hoxd13a or hoxa13a mRNA, shh expression was observed at 22.5 hpf.
Temporal shift of Shh activity leads to morphological changes in endoskeletal elements of pectoral fins in zebrafish and dogfish
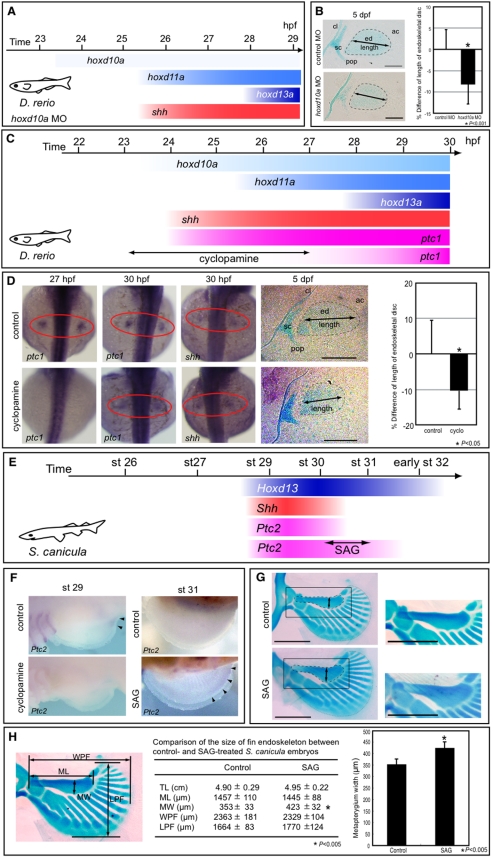
We next investigated whether a change in the timing of onset of shh expression induced by injection of hoxd10a MO could lead to a change in the zebrafish pectoral fin morphology (Fig. 5A and B). Zebrafish pectoral fins consist of an scapulocoracoid, a post-coracoid process, an endoskeletal disc, and actinotrichs at 5 days post-fertilization (dpf) [36]. Embryos were fixed and stained with Alcian Blue. Measurement of the endoskeletal discs of embryos injected with hoxd10a MO revealed that the total length of the disc along the proximal-distal axis was 8.41% shorter (P<0.001) compared with controls (control embryos, n = 8; hoxd10a MO injected embryos, n = 16; Fig. 5B).
Figure 5. Temporal shift of Shh activity leads to changes in pectoral fin morphology.
(A) shh expression appears at 25.5 hpf in pectoral fin primordia of D. rerio embryos injected with 5 ng of hoxd10a MO. (B) At 5 dpf, pectoral fins of embryos injected with control MO or with hoxd10a MO were stained with Alcian Blue (left). Cleithrum (cl), scapulocoracoid (sc), postcoracoid process (pop), endoskeletal disc (ed) and actinotrichs (ac) are indicated. Scale bars: 100 µm. The relative lengths of the endoskeletal disc are presented in the graph (right). *P<0.001, as assessed by Student's t-test. (C) shh expression appears at 24 hpf, concomitantly with hoxd10a, in pectoral fin primordia of D. rerio. Hedgehog signaling was blocked by treatment with 60 µM cyclopamine from 23 to 27 hpf, resulting in ablation of ptc1 expression until at least 27 hpf. ptc1 expression was recovered by 30 hpf in pectoral fin primordia of cyclopamine-treated embryos. (D) ptc1 and shh expression were examined in control or cyclopamine-treated embryos at the indicated stages (left). At 5 dpf, pectoral fins of control or cyclopamine-treated embryos were stained with Alcian Blue (middle). Scale bars: 200 µm. The relative lengths of the endoskeletal disc are represented in a graph (right). *P<0.05, as assessed by Student's t-test with Welch's correction. (E) Shh and Ptc2 expression disappeared before stage 31 in pectoral fin buds of S. canicula embryos. Hedgehog signaling was extended by treatment with SAG for 6 days from stage 30 to 31, resulting in extension of Ptc2 expression until at least stage 31. (F) Ptc2 expression was examined in control or SAG-treated embryos at stage 31 (5 days after the initial treatment). (G) Pectoral fins of control or SAG-treated embryos were stained with Alcian Blue. Anterior is to the left. Proximal is to the top. Insets show magnified views of the pectoral fin metapterygium. Note that the width of the metapterygium (arrows) of SAG-treated embryos was significantly increased. Scale bars: 1 mm. (H) Comparison of the size of the pectoral fin endoskeleton between control and SAG-treated S. canicula embryos. The table shows the total body length (TL), metapterygium length (ML), metapterygium width (MW), width across the base of pectoral fin endoskeleton (WPF), and length of pectoral fin endoskeleton (LPF) of control and SAG-treated embryos. The metapterygium lengths are represented in the bar graph. *P<0.05, as assessed by Student's t-test.
To confirm that a change in the timing of Shh activity during fin development could modify fin size, we treated embryos between 23 and 27 hpf with 60 µM cyclopamine, a steroidal alkaloid that inhibits hh signal transduction (Fig. 5C and D). Control embryos showed expression of ptc1, a marker for the primary targets of hh signaling, in the posterior margin of fin primordia at 27 hpf and 30 hpf (n = 4 and 5, respectively, Fig. 5D). Expression of ptc1 in cyclopamine-treated embryos was barely detectable in the fin primordia at 27 hpf (n = 5, Fig. 5D), whereas posterior activation of ptc1 was readily detectable by 30 hpf (n = 9, Fig. 5D), indicating that cyclopamine treatment efficiently blocked hh signaling through 27 hpf. Thus, stimulation of an artificial heterochronic shift of Shh activity in the pectoral fin primordia was successful. Expression levels of shh, which are upregulated by a feedback loop of hh signaling, were normal in fin primordia of either ethanol- or cyclopamine-treated embryos at 30 hpf, indicating that Shh activity itself is not required for maintenance of shh expression between 23 and 30 hpf. Taken together, the results indicate that shh signal transduction was efficiently blocked in fin primordia of embryos treated with cyclopamine until 27 hpf, but signaling was recovered at least by 30 hpf. To examine the fin morphology at 5 dpf, embryos were fixed and stained with Alcian Blue. Measurements of the cyclopamine-treated endoskeletal discs revealed that the total length of the disc along the proximal-distal axis was 10.6% shorter than those of controls (ethanol-treated embryos, n = 7; cyclopamine-treated embryos, n = 9). The difference in the length between the ethanol- and cyclopamine-treated discs was significant at 0.05 levels by Student's t-test with Welch's correction (Fig. 5D). A longer exposure with cyclopamine until 57 hpf resulted in a more severe reduction (19.4%) in the length of the endoskeletal disc (ethanol-treated embryos, n = 7; cyclopamine-treated embryos, n = 9; Fig. S5). This reduction seemed to be depend on both the apical fold activity and shh activity [16]. These results indicate that the temporal shift of the onset of shh expression in pectoral fin primordia can lead to a change in the size of the endoskeletal discs along the proximal-distal axis in zebrafish embryos.
We then investigated whether hh signaling can be manipulated in pectoral fins of dogfish embryos (Fig. 5E–H). Prior to the treatment of dogfish embryos with SAG, agonists of smoothened [37], we tested whether SAG is applicable in live embryos using zebrafish and confirmed that we could manipulate hh activity by treatment with SAG (Fig. S5C). We then treated dogfish embryos with cyclopamine or SAG to test whether such treatment could modify hh signaling in developing dogfish embryos. At stage 29, Ptc2 expression was observed in the posterior margin of pectoral fins of control embryos, whereas no Ptc2 transcripts were detected in pectoral fins of cyclopamine-treated embryos (Fig. 5E). On the other hand, treatment with SAG resulted in extensive Ptc2 expression in pectoral fins at stage 31 (Fig. 5E). These data demonstrated that hh signaling could be directly manipulated in dogfish embryos during fin development.
To examine whether the heterochronic shift of hh activity could alter the morphology of dogfish pectoral fins, we reared SAG-treated dogfish embryos for 11 to 12 weeks and then stained them with Alcian Blue. For SAG-treated embryos (n = 8), the width of the metapterygium was 19.8% greater compared with control embryos (n = 6; Fig. 5G, H). The difference in the metapterygium width between the control- and SAG-treated discs was significant by the Student's t-test with Welch's correction (P<0.005; Fig. 5H).
Taken together, our results indicate that altering the threshold levels of hox transcripts can trigger a heterochronic shift of shh expression in pectoral fin primordia, and the subsequent temporal shift of Shh activity causes changes in the size of the fin endoskeleton.
Discussion
Our investigation of the genetic basis of vertebrate morphological evolution has yielded the following findings. (1) Shh expression appears as soon as there is a morphological bud in mouse and chick embryos (concomitant with Hoxd10), whereas Shh is transcribed very late (concomitant with Hoxd13) in pectoral fin buds of dogfish (S. canicula). (2) A threshold level of accumulated hox transcripts is critical for the timing of shh expression; specifically, if the amount of hoxd10a transcripts is below a threshold level, shh expression does not appear until hoxd11a is expressed in zebrafish. (3) A quantitative change of hox transcripts leads to changes in the size of the zebrafish endoskeleton. (4) A temporal shift in Shh activation in paired fins leads to a change in endoskeleton size in both dogfish and zebrafish.
Heterochronic shift of Shh transcriptional onset depends on the quantity of Hox
Examination of collinear 5′-located Hoxa and Hoxd expression revealed that Shh expression was turned on when Hoxd13 expression appeared, concomitant with a further increase in 5′-located Hoxa and Hoxd expression. These results raise the possibility that the late onset of Shh transcription in the pectoral fins of S. canicula embryos might correlate with either specific Hox transcripts or the overall expression level of Hox transcripts. Using zebrafish embryos, which allowed us to alter the levels of specific hox transcripts, we showed that the onset of shh expression is controlled by a certain threshold level of accumulated 5′-located hox transcripts. A recent study using Hoxa/Hoxd double mutant mice showed that there is a boundary between Hoxd9, the last Hox unable to elicit Shh transcription, and Hoxd10, the first Hox to activate Shh [3] —that is, between the genes expressed throughout the limb bud and those excluded from the anterior region. The authors proposed that the limb anterior-posterior polarity arises from the co-option of the collinear Hox gene expression across the main body axis [3]. Importantly, our experiments in dogfish showed that Shh transcripts do not appear until the onset of Hoxd13 expression regardless of the nested posterior expression of Hoxd10–12. In other words, the Shh does not always initiate its expression even when the three penultimate Hoxd genes have already expressed posteriorly in paired appendages. The combination of experiments using both dogfish and zebrafish embryos has demonstrated that 5′-located Hox transcripts may not always reach the threshold levels required to stimulate Shh expression, even when the last four Hox paralog groups are expressed posteriorly. Absolute quantification of Hox gene transcripts necessary for Shh activation in mouse limb buds and in dogfish fin buds would allow us to further characterize the mechanisms by which Hox gene expression thresholds contribute to the evolution of vertebrate paired appendages. Although currently threre are no cartilaginous fishes amenable to transgenics manipulation or MO/mRNA injection, the prospective manipulation of Hox expression levels in these primitive gnathostomes should provide direct insight into our hypothesis of paired appendage evolution.
Hox and co-factors in heterochronic shift of Shh activation
During vertebrate evolution, quantitative changes in Hox expression, Hox cofactors, and/or other unknown factors, could have shifted the onset of Shh expression, leading to changes in the morphology of endoskeleton. Hox genes act partially through the aid of co-factors, such as Meis and Pbx [38]. Although 5′-located Hox genes have been shown to act through Meis, we found that only Pbx2 expression overlapped with Shh expression in pectoral fins in dogfish embryos (Fig. S6). Furthermore, manipulation of the level of pbx2 expression in zebrafish embryos resulted in a change in the timing of the onset of shh expression in pectoral fin primordia in a low percentage of embryos (see Fig. S4 and S6). This may be due to a low level of hox in pectoral fin primordia. Alternatively, Pbx may make a smaller contribution than Hox to the activation of Shh expression. Biochemical approaches that address the roles of Hox co-factors in the onset of Shh expression will provide new insights into vertebrate limb evolution.
Signalling pathways that control Hox expression levels
Signalling that regulates Hox transcriptional activation has been studied intensively. Retinoic acid is one of the factors thought to play key roles in controlling Hox gene transcription [39], [40], [41]. In zebrafish, a lack of retinoic acid in the pectoral fin buds results in the downregulation of shh, hoxd11 and hoxd12 [42]. In mice lacking retinoic acid-synthesizing enzyme gene–retinaldehyde dehydrogenase 2 (Raldh2), Shh expression is greatly reduced in the limb buds and seen along the distal margin, whereas Hoxd11 and Hoxd12 are ectopically expressed in early limb buds [43]. Hox genes are differentially activated by retinoic acid in a concentration-dependent manner and in a sequential order that is collinear with their 3′ to 5′ arrangement in the cluster [44]. It would be interesting to explore whether retinoic acid reaches levels sufficient to activate 5′Hoxd genes at different times in the posterior paired appendages between dogfish and other tetrapods.
The zinc finger transcriptional factor GLI3 is another protein known to modulate Hox expression. In early limb buds of mouse embryos, GLI3 negatively regulates the expression of 5′-located Hoxd genes [45], [46]. In mouse and chick embryos, Gli3 expression is excluded from the posterior part of the limb buds, when Hand2 expression appears in the posterior region. Gli3, in turn, restricts Hand2 expression in the posterior limb buds [47]. Such reciprocal antagonism seems to have been established in cartilaginous fishes, as Hand2 expression is restricted to the posterior part of pectoral fins in S. canicula [20], indicating GLI3 may be involved in regulating Hox expression in the posterior region of dogfish fins. In addition, GLI3 physically interacts with HOXD12 during digit patterning [48]. In this regard, comparative analysis of the expression and function of Gli3 with respect to Hox expression would enhance our understanding of the evolution of genetic networks involved in regulating Shh expression.
Heterochronic shift of Shh onset in vertebrate fin evolution
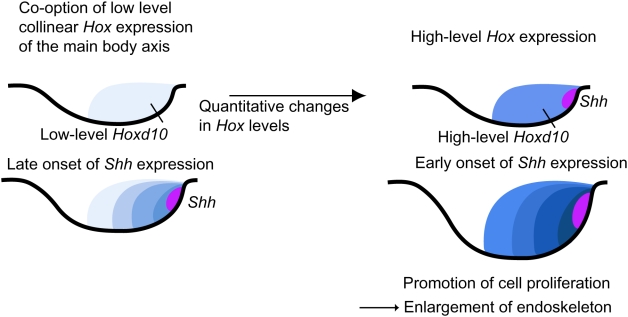
Our results provide new clues for understanding the sequential events of vertebrate fin/limb evolution, especially with respect to the molecular mechanisms that change the onset of shh expression and lead to morphological changes in endoskeletal components (Fig. 6). It has been proposed that paired appendages adopted collinear expression of Hox from the main body axis concomitant with their emergence in the body wall [3], [49], [50] (Fig. 6). Our results suggest that if threshold levels of accumulated 5′ Hox transcripts were not reached, Shh expression may have been delayed or silent in ancestral fin buds. Quantitative changes in accumulated 5′ Hox may have led to altered onset of Shh expression, resulting in enlargement of endoskeletal elements during fin evolution (Fig. 6).
Figure 6. Diagram representing the effect of Shh expression heterochrony on vertebrate paired appendage evolution.
A model suggesting that the early fin buds may have acquired low levels of Hox expression by co-option of collinear Hox expression in the main body axis [53]. Changes in accumulated Hox could have led to altered onset of Shh expression, resulting in enlargement of the endoskeletal elements during fin evolution.
Endoskeletal components of paired appendages during the transformation from fins into limbs have been throughly discussed. Comparison of the paired appendages in fossils and in living primitive sarcopterygian fishes (lobe-finned fishes including lungfish and coelacanths) showed that endoskeletal elements of the paired appendages increased in size prior to the acquisition of the digital plates. Thus, the transition from fins to limbs seems to have required at least two major events, namely the enlargement of proximal endoskeletal elements with subsequent acquisition of digital plates. It has been proposed that the transformation of the apical fin fold into the short, apical ectodermal ridge may have promoted endoskeletal proliferation [4]. Here, we demonstrated that a temporal shift in Shh activity could have also led to changes in the size of the endoskeletal elements along the proximal-distal axis.
The effects of the late onset of Shh expression on limb morphology are difficult to examine using chick or mouse embryos because loss of Shh activity disrupts the Fgf/Shh positive feedback loop [51]. To circumvent this problem, we have taken advantage of the pectoral fin primordia of zebrafish embryos, in which shh expression occurs prior to the formation of the apical ectodermal ridge–like structure. We showed that temporal block of Shh signaling by cyclopamine, an inhibitor of hh signaling, prior to apical ridge formation can lead to a reduction in the size of fin endoskeletal elements (Fig. 5). Treatment with cyclopamine did not alter shh expression levels in fin primordia, indicating that Shh signaling recovers from cyclopamine treatment prior to the formation of the Fgf/Shh positive feedback loop [51]. Consistent with our proposal, studies in the zebrafish sonic you (syu) mutant, in which shh is disrupted, showed that shh in the early pectoral fin buds promotes cell proliferation that is at least partially independent of the apical fold, because a reduction in cell proliferation in syu fin buds was seen prior to the reduction of the apical fin fold and of shh expression [16]. In pectoral fin buds of the syu mutant, a more severe reduction in fin bud size was seen after ablation of the apical fold [16]. In tetrapod limbs, Shh together with Fgfs promote overproliferation of the posterior mesenchymal cells, leading to asymmetric growth of the limb [20], [51], [52]. Furthermore, recent studies revealed that Shh signalling controls not only the specification of digit progenitors but also cell proliferation in limb buds of chick embryos [14]. Thus, the temporal shift of Shh expression during vertebrate fin/limb evolution could have acted independently of, and/or synergistically with, Fgf signals from the apical fold, which also shift the timing of folding and promote cell proliferation, thereby contributing to the formation of the endoskeleton.
Because zebrafish larval pectoral fins are later remodeled to form the adult pectoral fins, it is difficult to speculate which endoskeletal components of paired fins among primitive fishes may have been affected by temporal changes in shh expression during evolution. Furthermore, the metapterygium was lost in the teleost lineage. Therefore, examination of these features in the paired appendages of the primitive cartilaginous dogfish is highly informative. Although dogfish embryos did not survive beyond 2 weeks after cyclopamine treatment (presumably due to the multiple malformations; data not shown), we have succeeded in keeping them alive for 12 weeks after treatment with SAG (Fig. 5G, H). We showed that extension of Shh activity using SAG could enlarge the metapterygium of dogfish pectoral fins. The metapterygium, a proximal component of the dogfish fin, has been considered to have persisted in sarcopterygian fishes and was the ancestral structure from which the tetrapod limb evolved. Enlargement of the dogfish fin metapterygium by extending Shh activity indicates that Shh could have promoted the proliferation of cells that formed the proximal structures among ancestral species. We propose that a heterochronic shift of the onset of Shh expression could have been mediated by changes in the level of Hox (and Hox co-factors) and that such transcriptional heterochrony could have influenced the proliferation of cells that contributed to the formation of endoskeletal components during vertebrate paired appendage evolution (Fig. 6). It would not be surprising if such a system controls the morphological diversification of paired appendages in different lineages (including lineages of cartilaginous fishes). It will be interesting to characterize these features of the body plan among different vertebrates having various types of paired appendages.
Supporting Information
Expression of Shh and Fgfs during S. canicula fin development. (A) RT-PCR of ScShh in stage 29 and 32 S. canicula pectoral fin buds (left); results for stage 27 S. canicula embryos have been published [20]. The right panel shows semi-quantitative analysis of ScShh mRNA expression in pectoral fins relative to the ScGAPDH mRNA level. (B) Frontal view of the facial region at stage 27. (C–D, FG) Pectoral fin buds. Anterior is to the left. (B–D) ScFgf8 expression at stage 27 (B, C) and 32 (D). Although transcripts were observed in nasal pits (np) and gill filaments (gf), no transcripts were detected in the apical fin fold (aff). (E) RT-PCR of ScFgf8 in head (Head) and pectoral fins (Pec) of S. canicula embryos. (F) Staining of anti-Fgf4 antibody at stage 27. Arrowheads indicate anti-Fgf4-positive cells in the apical fin fold. (G) Scβ-catenin expression at stage 32. Abundant Scβ-catenin transcripts in pectoral fins including the apical fin fold (arrowheads) demonstrates probe efficacy.
(8.68 MB TIF)
Expression of hoxd10a, hoxd11a and hoxd13a during D. rerio pectoral fin development. Dorsal view of embryos injected with 5 ng of the control morpholino (MO) at 24, 25.5, 27 and 28 hpf. Red ovals highlight the pectoral fin primordia. Expression of hoxd10a was initially detected at 24 hpf, hoxd11a at 25.5 hpf, and hoxd13a at 28 hpf.
(8.08 MB TIF)
Quantitative PCR analyses of hoxd11a and shh expression in the lateral plate mesoderm of zebrafish embryos. Levels of hoxd11a (A) and shh (B–D) mRNAs in the lateral plate mesoderm of embryos were quantified relative to the gapdh mRNA level. (A–D) Expression levels of hoxd11a (A) and shh (B, C) in the lateral plate mesoderm of 24 hpf (B) and 25.5 hpf (A, C) embryos injected with 5 ng control, 1 ng hoxd10a, or 2.5 ng hoxd10a MO. (D) Quantitative PCR analyses to determine the expression levels of shh in the lateral plate mesoderm of embryos injected with 5 ng control MO, 5 pg hoxd10a mRNA, or 20 pg hoxd10a mRNA. Expression of shh was undetectable by quantitative PCR in 22.5 hpf injected with 5 ng control MO.
(2.32 MB TIF)
Onset of shh expression in zebrafish embryo fin primordia primarily depends on hox expression. The percentage of D. rerio embryos expressing the indicated level of shh transcript at 22.5, 24, or 25.5 hpf following injection of the indicated amount of control MO, hoxd10a MO, hoxd13a MO, hoxd10a mRNA, hoxd13a mRNA, hoxa13a mRNA, pbx2 MO or pbx2 mRNA is shown. A representative image depicting the detectable or undetectable levels of shh expression in the pectoral fin primordia is shown in Figure 2D.
(0.44 MB EPS)
Treatment of zebrafish embryos with cyclopamine or SAG. (A) Hedgehog signaling was blocked by treatment with 60 µM cyclopamine from 23 to 57 hpf, resulting in ablation of ptc1 expression until at least 60 hpf. ptc1 expression recovered by 72 hpf in pectoral fin primordia of cyclopamine-treated embryos. (B) ptc1 expression was examined in control or cyclopamine-treated embryos at the indicated stages (left). At 5 dpf, pectoral fins of control (n = 7) or cyclopamine-treated embryos (n = 9) were stained with Alcian Blue (middle). The relative lengths of the endoskeletal disc are presented in the graph (right). *P<10−6, as assessed by Student's t-test. Cleithrum (cl), scapulocoracoid (sc), postcoracoid process (pop), endoskeletal disc (ed) and actinotrichs (ac) are indicated. Scale bars: 200 µm. (C) Zebrafish embryos were treated with SAG or cyclopamine, and ptc1 expression was examined in adaxial cells. The specification of adaxial cells is known to depend on Hh signaling [1]. Panels show the dorsal view of ptc1 expression in an 8-somite-stage control embryo (left), in a SAG-treated embryo (middle), and in a cyclopamine-treated embryo (right). In control embryo, adaxial cells are indicated by brackets. Note that ptc1 expression is expanded in the SAG-treated embryo (brackets), whereas it is undetectable in the cyclopamine-treated embryo (right). 1. Wolff C, Roy S, Ingham PW (2003) Multiple muscle cell identities induced by distinct levels and timing of hedgehog activity in the zebrafish embryo. Curr Biol 13: 1169–1181.
(7.33 MB TIF)
The hox co-factor pbx makes a lesser contribution than hox to the onset of shh expression. (A) Expression of Meis1 and Pbx2 in the pectoral fin of S. canicula embryos at the indicated stages (top panels). Anterior is to the left. Arrowheads indicate transcripts in the proximal region. (B) Left: representative images depicting the detectability of shh expression in the pectoral fin primordia. Right: the percentage of D. rerio embryos with the indicated level of shh expression observed at 22.5, 24, and 25.5 hpf following injection of control MO, pbx2 MO, or pbx2 mRNA.
(9.69 MB TIF)
Acknowledgments
We thank C. Tickle for her generous support and helpful comments. We also thank A. A. W. Tweedale for collecting S. canicula embryos, E. Tiecke and A. Bain for fixing embryos, Y. Murata, Y. Aita and K. Yoshida for technical assistance, S. Hirose, M. Okabe, K. Shirahige and N. Okada for allowing us to use their facilities, and K. Hoshijima for technical advice.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported by a Grant-in-Aid for Young Scientists (A) from the Ministry of Education, Science, Sports and Culture of Japan, by a Tokyo Tech Award for Challenging Research and by a grant from the Hayashi Memorial Foundation for Female Natural Scientists. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Sordino P, van der Hoeven F, Duboule D. Hox gene expression in teleost fins and the origin of vertebrate digits. Nature. 1995;375:678–681. doi: 10.1038/375678a0. [DOI] [PubMed] [Google Scholar]
- 2.Kmita M, Tarchini B, Zakany J, Logan M, Tabin CJ, et al. Early developmental arrest of mammalian limbs lacking HoxA/HoxD gene function. Nature. 2005;435:1113–1116. doi: 10.1038/nature03648. [DOI] [PubMed] [Google Scholar]
- 3.Tarchini B, Duboule D, Kmita M. Regulatory constraints in the evolution of the tetrapod limb anterior-posterior polarity. Nature. 2006;443:985–988. doi: 10.1038/nature05247. [DOI] [PubMed] [Google Scholar]
- 4.Thorogood P. The development of the Teleost fin and implications for our understanding of tetrapod limb evolution. In: Hinchliffe JR, Hurle JM, Summerbell D, editors. Developmental Patterning of the Vertebrate Limb. New York: Plenum Pub Corp; 1991. [Google Scholar]
- 5.Saunders JW, Jr, Gasseling MT. Ectoderm-mesenchymal interaction in the origin of wing symmetry. In: Fleischmajer R, Billingham RE, editors. Epithelial–Mesenchymal Interactions. Baltimore: Williams and Wilkins; 1968. pp. 78–97. [Google Scholar]
- 6.Tickle C, Summerbell D, Wolpert L. Positional signalling and specification of digits in chick limb morphogenesis. Nature. 1975;254:199–202. doi: 10.1038/254199a0. [DOI] [PubMed] [Google Scholar]
- 7.Tickle C. The number of polarizing region cells required to specify additional digits in the developing chick wing. Nature. 1981;289:295–298. doi: 10.1038/289295a0. [DOI] [PubMed] [Google Scholar]
- 8.Riddle RD, Johnson RL, Laufer E, Tabin C. Sonic hedgehog mediates the polarizing activity of the ZPA. Cell. 1993;75:1401–1416. doi: 10.1016/0092-8674(93)90626-2. [DOI] [PubMed] [Google Scholar]
- 9.Echelard Y, Epstein DJ, St-Jacques B, Shen L, Mohler J, et al. Sonic hedgehog, a member of a family of putative signaling molecules, is implicated in the regulation of CNS polarity. Cell. 1993;75:1417–1430. doi: 10.1016/0092-8674(93)90627-3. [DOI] [PubMed] [Google Scholar]
- 10.Krauss S, Concordet JP, Ingham PW. A functionally conserved homolog of the Drosophila segment polarity gene hh is expressed in tissues with polarizing activity in zebrafish embryos. Cell. 1993;75:1431–1444. doi: 10.1016/0092-8674(93)90628-4. [DOI] [PubMed] [Google Scholar]
- 11.Yang Y, Drossopoulou G, Chuang PT, Duprez D, Marti E, et al. Relationship between dose, distance and time in Sonic Hedgehog-mediated regulation of anteroposterior polarity in the chick limb. Development. 1997;124:4393–4404. doi: 10.1242/dev.124.21.4393. [DOI] [PubMed] [Google Scholar]
- 12.Harfe BD, Scherz PJ, Nissim S, Tian H, McMahon AP, et al. Evidence for an expansion-based temporal Shh gradient in specifying vertebrate digit identities. Cell. 2004;118:517–528. doi: 10.1016/j.cell.2004.07.024. [DOI] [PubMed] [Google Scholar]
- 13.Scherz PJ, McGlinn E, Nissim S, Tabin CJ. Extended exposure to Sonic hedgehog is required for patterning the posterior digits of the vertebrate limb. Dev Biol. 2007;308:343–354. doi: 10.1016/j.ydbio.2007.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Towers M, Mahood R, Yin Y, Tickle C. Integration of growth and specification in chick wing digit-patterning. Nature. 2008;452:882–886. doi: 10.1038/nature06718. [DOI] [PubMed] [Google Scholar]
- 15.Zhu J, Nakamura E, Nguyen MT, Bao X, Akiyama H, et al. Uncoupling Sonic hedgehog control of pattern and expansion of the developing limb bud. Dev Cell. 2008;14:624–632. doi: 10.1016/j.devcel.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Neumann CJ, Grandel H, Gaffield W, Schulte-Merker S, Nusslein-Volhard C. Transient establishment of anteroposterior polarity in the zebrafish pectoral fin bud in the absence of sonic hedgehog activity. Development. 1999;126:4817–4826. doi: 10.1242/dev.126.21.4817. [DOI] [PubMed] [Google Scholar]
- 17.Dahn RD, Davis MC, Pappano WN, Shubin NH. Sonic hedgehog function in chondrichthyan fins and the evolution of appendage patterning. Nature. 2007;445:311–314. doi: 10.1038/nature05436. [DOI] [PubMed] [Google Scholar]
- 18.Charite J, McFadden DG, Olson EN. The bHLH transcription factor dHAND controls Sonic hedgehog expression and establishment of the zone of polarizing activity during limb development. Development. 2000;127:2461–2470. doi: 10.1242/dev.127.11.2461. [DOI] [PubMed] [Google Scholar]
- 19.Crossley PH, Minowada G, MacArthur CA, Martin GR. Roles for FGF8 in the induction, initiation, and maintenance of chick limb development. Cell. 1996;84:127–136. doi: 10.1016/s0092-8674(00)80999-x. [DOI] [PubMed] [Google Scholar]
- 20.Tanaka M, Munsterberg A, Anderson WG, Prescott AR, Hazon N, et al. Fin development in a cartilaginous fish and the origin of vertebrate limbs. Nature. 2002;416:527–531. doi: 10.1038/416527a. [DOI] [PubMed] [Google Scholar]
- 21.Maruoka Y, Ohbayashi N, Hoshikawa M, Itoh N, Hogan BL, et al. Comparison of the expression of three highly related genes, Fgf8, Fgf17 and Fgf18, in the mouse embryo. Mech Dev. 1998;74:175–177. doi: 10.1016/s0925-4773(98)00061-6. [DOI] [PubMed] [Google Scholar]
- 22.Niswander L, Martin GR. Fgf-4 expression during gastrulation, myogenesis, limb and tooth development in the mouse. Development. 1992;114:755–768. doi: 10.1242/dev.114.3.755. [DOI] [PubMed] [Google Scholar]
- 23.Niswander L, Tickle C, Vogel A, Booth I, Martin GR. FGF-4 replaces the apical ectodermal ridge and directs outgrowth and patterning of the limb. Cell. 1993;75:579–587. doi: 10.1016/0092-8674(93)90391-3. [DOI] [PubMed] [Google Scholar]
- 24.Sun X, Mariani FV, Martin GR. Functions of FGF signalling from the apical ectodermal ridge in limb development. Nature. 2002;418:501–508. doi: 10.1038/nature00902. [DOI] [PubMed] [Google Scholar]
- 25.Zakany J, Kmita M, Duboule D. A dual role for Hox genes in limb anterior-posterior asymmetry. Science. 2004;304:1669–1672. doi: 10.1126/science.1096049. [DOI] [PubMed] [Google Scholar]
- 26.Capellini TD, Di Giacomo G, Salsi V, Brendolan A, Ferretti E, et al. Pbx1/Pbx2 requirement for distal limb patterning is mediated by the hierarchical control of Hox gene spatial distribution and Shh expression. Development. 2006;133:2263–2273. doi: 10.1242/dev.02395. [DOI] [PubMed] [Google Scholar]
- 27.Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. Stages of embryonic development of the zebrafish. Dev Dyn. 1995;203:253–310. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- 28.Westerfield M. A guide for the laboratory use of zebrafish (Danio rerio). The zebrafish book. Eugene: University of Oregon Press; 2000. [Google Scholar]
- 29.Horigome N, Myojin M, Ueki T, Hirano S, Aizawa S, et al. Development of cephalic neural crest cells in embryos of Lampetra japonica, with special reference to the evolution of the jaw. Dev Biol. 1999;207:287–308. doi: 10.1006/dbio.1998.9175. [DOI] [PubMed] [Google Scholar]
- 30.Waskiewicz AJ, Rikhof HA, Moens CB. Eliminating zebrafish pbx proteins reveals a hindbrain ground state. Dev Cell. 2002;3:723–733. doi: 10.1016/s1534-5807(02)00319-2. [DOI] [PubMed] [Google Scholar]
- 31.Thisse B, Heyer V, Lux A, Alunni V, Degrave A, et al. Spatial and temporal expression of the zebrafish genome by large-scale in situ hybridization screening. Methods Cell Biol. 2004;77:505–519. doi: 10.1016/s0091-679x(04)77027-2. [DOI] [PubMed] [Google Scholar]
- 32.Covassin L, Amigo JD, Suzuki K, Teplyuk V, Straubhaar J, et al. Global analysis of hematopoietic and vascular endothelial gene expression by tissue specific microarray profiling in zebrafish. Dev Biol. 2006;299:551–562. doi: 10.1016/j.ydbio.2006.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kimmel CB, Miller CT, Kruze G, Ullmann B, BreMiller RA, et al. The shaping of pharyngeal cartilages during early development of the zebrafish. Dev Biol. 1998;203:245–263. doi: 10.1006/dbio.1998.9016. [DOI] [PubMed] [Google Scholar]
- 34.Davis MC, Dahn RD, Shubin NH. An autopodial-like pattern of Hox expression in the fins of a basal actinopterygian fish. Nature. 2007;447:473–476. doi: 10.1038/nature05838. [DOI] [PubMed] [Google Scholar]
- 35.Freitas R, Zhang G, Cohn MJ. Biphasic Hoxd gene expression in shark paired fins reveals an ancient origin of the distal limb domain. PLoS ONE. 2007;2:e754. doi: 10.1371/journal.pone.0000754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grandel H, Schulte-Merker S. The development of the paired fins in the zebrafish (Danio rerio). Mech Dev. 1998;79:99–120. doi: 10.1016/s0925-4773(98)00176-2. [DOI] [PubMed] [Google Scholar]
- 37.Chen JK, Taipale J, Young KE, Maiti T, Beachy PA. Small molecule modulation of Smoothened activity. Proc Natl Acad Sci U S A. 2002;99:14071–14076. doi: 10.1073/pnas.182542899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Burglin TR. Analysis of TALE superclass homeobox genes (MEIS, PBC, KNOX, Iroquois, TGIF) reveals a novel domain conserved between plants and animals. Nucleic Acids Res. 1997;25:4173–4180. doi: 10.1093/nar/25.21.4173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Serpente P, Tumpel S, Ghyselinck NB, Niederreither K, Wiedemann LM, et al. Direct crossregulation between retinoic acid receptor {beta} and Hox genes during hindbrain segmentation. Development. 2005;132:503–513. doi: 10.1242/dev.01593. [DOI] [PubMed] [Google Scholar]
- 40.Gould A, Itasaki N, Krumlauf R. Initiation of rhombomeric Hoxb4 expression requires induction by somites and a retinoid pathway. Neuron. 1998;21:39–51. doi: 10.1016/s0896-6273(00)80513-9. [DOI] [PubMed] [Google Scholar]
- 41.Huang D, Chen SW, Langston AW, Gudas LJ. A conserved retinoic acid responsive element in the murine Hoxb-1 gene is required for expression in the developing gut. Development. 1998;125:3235–3246. doi: 10.1242/dev.125.16.3235. [DOI] [PubMed] [Google Scholar]
- 42.Gibert Y, Gajewski A, Meyer A, Begemann G. Induction and prepatterning of the zebrafish pectoral fin bud requires axial retinoic acid signaling. Development. 2006;133:2649–2659. doi: 10.1242/dev.02438. [DOI] [PubMed] [Google Scholar]
- 43.Niederreither K, Vermot J, Schuhbaur B, Chambon P, Dolle P. Embryonic retinoic acid synthesis is required for forelimb growth and anteroposterior patterning in the mouse. Development. 2002;129:3563–3574. doi: 10.1242/dev.129.15.3563. [DOI] [PubMed] [Google Scholar]
- 44.Simeone A, Acampora D, Arcioni L, Andrews PW, Boncinelli E, et al. Sequential activation of HOX2 homeobox genes by retinoic acid in human embryonal carcinoma cells. Nature. 1990;346:763–766. doi: 10.1038/346763a0. [DOI] [PubMed] [Google Scholar]
- 45.Buscher D, Bosse B, Heymer J, Ruther U. Evidence for genetic control of Sonic hedgehog by Gli3 in mouse limb development. Mech Dev. 1997;62:175–182. doi: 10.1016/s0925-4773(97)00656-4. [DOI] [PubMed] [Google Scholar]
- 46.Zuniga A, Zeller R. Gli3 (Xt) and formin (ld) participate in the positioning of the polarising region and control of posterior limb-bud identity. Development. 1999;126:13–21. doi: 10.1242/dev.126.1.13. [DOI] [PubMed] [Google Scholar]
- 47.te Welscher P, Fernandez-Teran M, Ros MA, Zeller R. Mutual genetic antagonism involving GLI3 and dHAND prepatterns the vertebrate limb bud mesenchyme prior to SHH signaling. Genes Dev. 2002;16:421–426. doi: 10.1101/gad.219202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen Y, Knezevic V, Ervin V, Hutson R, Ward Y, et al. Direct interaction with Hoxd proteins reverses Gli3-repressor function to promote digit formation downstream of Shh. Development. 2004;131:2339–2347. doi: 10.1242/dev.01115. [DOI] [PubMed] [Google Scholar]
- 49.Coates MI. Hox genes, fin folds and symmetry. Nature. 1993;364:195–196. [Google Scholar]
- 50.Thorogood P, Ferretti P. Hox gene, fin folds and symmetry. Nature. 1993;364:196. [Google Scholar]
- 51.Niswander L, Jeffrey S, Martin GR, Tickle C. A positive feedback loop coordinates growth and patterning in the vertebrate limb. Nature. 1994;371:609–612. doi: 10.1038/371609a0. [DOI] [PubMed] [Google Scholar]
- 52.Laufer E, Nelson CE, Johnson RL, Morgan BA, Tabin C. Sonic hedgehog and Fgf-4 act through a signaling cascade and feedback loop to integrate growth and patterning of the developing limb bud. Cell. 1994;79:993–1003. doi: 10.1016/0092-8674(94)90030-2. [DOI] [PubMed] [Google Scholar]
- 53.Dolle P, Izpisua-Belmonte JC, Falkenstein H, Renucci A, Duboule D. Coordinate expression of the murine Hox-5 complex homoeobox-containing genes during limb pattern formation. Nature. 1989;342:767–772. doi: 10.1038/342767a0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Expression of Shh and Fgfs during S. canicula fin development. (A) RT-PCR of ScShh in stage 29 and 32 S. canicula pectoral fin buds (left); results for stage 27 S. canicula embryos have been published [20]. The right panel shows semi-quantitative analysis of ScShh mRNA expression in pectoral fins relative to the ScGAPDH mRNA level. (B) Frontal view of the facial region at stage 27. (C–D, FG) Pectoral fin buds. Anterior is to the left. (B–D) ScFgf8 expression at stage 27 (B, C) and 32 (D). Although transcripts were observed in nasal pits (np) and gill filaments (gf), no transcripts were detected in the apical fin fold (aff). (E) RT-PCR of ScFgf8 in head (Head) and pectoral fins (Pec) of S. canicula embryos. (F) Staining of anti-Fgf4 antibody at stage 27. Arrowheads indicate anti-Fgf4-positive cells in the apical fin fold. (G) Scβ-catenin expression at stage 32. Abundant Scβ-catenin transcripts in pectoral fins including the apical fin fold (arrowheads) demonstrates probe efficacy.
(8.68 MB TIF)
Expression of hoxd10a, hoxd11a and hoxd13a during D. rerio pectoral fin development. Dorsal view of embryos injected with 5 ng of the control morpholino (MO) at 24, 25.5, 27 and 28 hpf. Red ovals highlight the pectoral fin primordia. Expression of hoxd10a was initially detected at 24 hpf, hoxd11a at 25.5 hpf, and hoxd13a at 28 hpf.
(8.08 MB TIF)
Quantitative PCR analyses of hoxd11a and shh expression in the lateral plate mesoderm of zebrafish embryos. Levels of hoxd11a (A) and shh (B–D) mRNAs in the lateral plate mesoderm of embryos were quantified relative to the gapdh mRNA level. (A–D) Expression levels of hoxd11a (A) and shh (B, C) in the lateral plate mesoderm of 24 hpf (B) and 25.5 hpf (A, C) embryos injected with 5 ng control, 1 ng hoxd10a, or 2.5 ng hoxd10a MO. (D) Quantitative PCR analyses to determine the expression levels of shh in the lateral plate mesoderm of embryos injected with 5 ng control MO, 5 pg hoxd10a mRNA, or 20 pg hoxd10a mRNA. Expression of shh was undetectable by quantitative PCR in 22.5 hpf injected with 5 ng control MO.
(2.32 MB TIF)
Onset of shh expression in zebrafish embryo fin primordia primarily depends on hox expression. The percentage of D. rerio embryos expressing the indicated level of shh transcript at 22.5, 24, or 25.5 hpf following injection of the indicated amount of control MO, hoxd10a MO, hoxd13a MO, hoxd10a mRNA, hoxd13a mRNA, hoxa13a mRNA, pbx2 MO or pbx2 mRNA is shown. A representative image depicting the detectable or undetectable levels of shh expression in the pectoral fin primordia is shown in Figure 2D.
(0.44 MB EPS)
Treatment of zebrafish embryos with cyclopamine or SAG. (A) Hedgehog signaling was blocked by treatment with 60 µM cyclopamine from 23 to 57 hpf, resulting in ablation of ptc1 expression until at least 60 hpf. ptc1 expression recovered by 72 hpf in pectoral fin primordia of cyclopamine-treated embryos. (B) ptc1 expression was examined in control or cyclopamine-treated embryos at the indicated stages (left). At 5 dpf, pectoral fins of control (n = 7) or cyclopamine-treated embryos (n = 9) were stained with Alcian Blue (middle). The relative lengths of the endoskeletal disc are presented in the graph (right). *P<10−6, as assessed by Student's t-test. Cleithrum (cl), scapulocoracoid (sc), postcoracoid process (pop), endoskeletal disc (ed) and actinotrichs (ac) are indicated. Scale bars: 200 µm. (C) Zebrafish embryos were treated with SAG or cyclopamine, and ptc1 expression was examined in adaxial cells. The specification of adaxial cells is known to depend on Hh signaling [1]. Panels show the dorsal view of ptc1 expression in an 8-somite-stage control embryo (left), in a SAG-treated embryo (middle), and in a cyclopamine-treated embryo (right). In control embryo, adaxial cells are indicated by brackets. Note that ptc1 expression is expanded in the SAG-treated embryo (brackets), whereas it is undetectable in the cyclopamine-treated embryo (right). 1. Wolff C, Roy S, Ingham PW (2003) Multiple muscle cell identities induced by distinct levels and timing of hedgehog activity in the zebrafish embryo. Curr Biol 13: 1169–1181.
(7.33 MB TIF)
The hox co-factor pbx makes a lesser contribution than hox to the onset of shh expression. (A) Expression of Meis1 and Pbx2 in the pectoral fin of S. canicula embryos at the indicated stages (top panels). Anterior is to the left. Arrowheads indicate transcripts in the proximal region. (B) Left: representative images depicting the detectability of shh expression in the pectoral fin primordia. Right: the percentage of D. rerio embryos with the indicated level of shh expression observed at 22.5, 24, and 25.5 hpf following injection of control MO, pbx2 MO, or pbx2 mRNA.
(9.69 MB TIF)