Table 2.
Palladium-catalyzed enyne cycloisomerizations
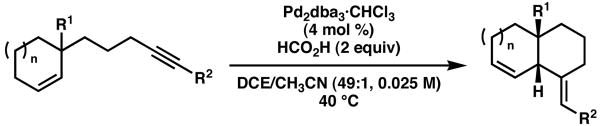 | |||||
|---|---|---|---|---|---|
| entry | R1 | R2 | n | yield, time (%, h)a | drb |
| 1 | CO2Me | CO2Me | 1 | 92, 4 | > 19:1 |
| 2 | CHO | CO2Me | 1 | 73, 5 | > 19:1 |
| 3 | CON(OMe)Me | CO2Me | 1 | 68, 15 | > 19:1 |
| 4 | CO2H | CO2Me | 1 | NR | -- |
| 5 | CH2OH | CO2Me | 1 | 83, 2.5 | > 19:1 |
| 6 | CO2Me | CO2Me | 2 | 95, 3 | > 19:1 |
| 7 | CHO | CO2Me | 2 | 92, 4 | > 19:1 |
| 8 | CON(OMe)Me | CO2Me | 2 | 95, 2.5 | > 19:1 |
| 9 | CO2Me | COi-Pr | 1 | 67, 12 | > 19:1 |
| 10 | CO2Me | CONEt2 | 1 | NR | -- |
Isolated yield.
Determined by 1H NMR.
