Abstract
Bacillus subtilis StoA is an extracytoplasmic thiol-disulfide oxidoreductase (TDOR) important for the synthesis of the endospore peptidoglycan cortex protective layer. Here we demonstrate that StoA is membrane-associated in B. subtilis and report the crystal structure of the soluble protein lacking its membrane anchor. This showed that StoA adopts a thioredoxin-like fold with N-terminal and internal additions that are characteristic of extracytoplasmic TDORs. The CXXC active site of the crystallized protein was found to be in a mixture of oxidized and reduced states, illustrating that there is little conformational variation between redox states. The midpoint reduction potential was determined as -248 mV versus normal hydrogen electrode at pH 7 consistent with StoA fulfilling a reductive role in endospore biogenesis. pKa values of the active site cysteines, Cys-65 and Cys-68, were determined to be 5.5 and 7.8. Although Cys-68 is buried within the structure, both cysteines were found to be accessible to cysteine-specific alkylating reagents. In vivo studies of site-directed variants of StoA revealed that the active site cysteines are functionally important, as is Glu-71, which lies close to the active site and is conserved in many reducing extracytoplasmic TDORs. The structure and biophysical properties of StoA are very similar to those of ResA, a B. subtilis extracytoplasmic TDOR involved in cytochrome c maturation, raising important general questions about how these similar but non-redundant proteins achieve specificity. A detailed comparison of the two proteins demonstrates that relatively subtle differences, largely located around the active sites of the proteins, are sufficient to confer specificity.
Bacteria of the genera Bacillus and Clostridium can form endospores in response to nutrient starvation. The endospore, which is a dormant and very resistant state of the bacterium, can germinate back into a vegetative cell once nutrients become available again. Different layers help to protect the endospore: the dehydrated core, corresponding to the cytoplasm and containing the genome, is surrounded by a peptidoglycan layer, the cortex, which is required for extreme heat resistance. Outside the cortex, coat layers of mainly proteins protect the endospore against damaging chemicals and enzymes (1). The StoA protein (also known as SpoIVH and YkvV) of Bacillus subtilis is a predicted membrane-bound thiol-disulfide oxidoreductase (TDOR)4 important for endospore cortex synthesis (2, 3). Inactivation of the stoA gene results in spores deficient in the cortex layer that are much more sensitive than wild-type spores to heat, lysozyme, and chloroform treatment.
TDORs are proteins that catalyze the reduction of disulfide bonds and the oxidation of thiols. One pair of cysteine residues, often found in a -CXXC-motif, is present in the active site of TDORs, and although TDORs generally lack high overall sequence similarity, many of them share a common three-dimensional fold called the thioredoxin fold (4). Within the cell under normal circumstances, TDORs preferentially exhibit either a reducing or an oxidizing function as determined, at least in part, by the reduction potential of their disulfide/thiol active site. Their function is essential for the stabilization, folding, and activity of many proteins in bacterial cells, and they are involved in a wide range of processes, including cytochrome synthesis, cell motility, natural competence development, and toxin biosynthesis (5-8). Known enzymes that function in bacterial cell wall peptidoglycan synthesis, e.g. transglycosidases and transpeptidases, do not depend on cysteine redox chemistry, and so an important role for StoA in cortex synthesis was unexpected. Studies of this protein can reveal hitherto unknown features of sporulation and peptidoglycan synthesis (9). From the primary sequence of StoA (see Fig. 1), it was predicted to have one transmembrane segment and a single membrane-extruded domain with a thioredoxin-like fold. It is therefore likely to function in the control of thiol disulfide chemistry of a substrate protein(s). In the absence of BdbD, which is an orthologue of Escherichia coli DsbA that catalyzes disulfide bond formation in proteins on the outside of the cytoplasmic membrane, StoA is no longer important for endospore cortex synthesis, indicating that it functions to specifically reduce disulfide bonds on the outside of the cytoplasmic membrane (2). It was also proposed that the protein most likely operates in the intermembrane space of the developing forespore where the cortex is synthesized. The substrate protein(s) of StoA with a function in cortex synthesis has not yet been identified, but the CcdA protein most likely functions in transmembrane transport of reducing equivalents from thioredoxin in the cytoplasm to StoA in the forespore intermembrane space (9, 10).
FIGURE 1.
Amino acid residue sequence alignment of B. subtilis sStoA used in structural and biochemical analyses, full-length StoA, and full-length ResA proteins. Invariant residues are marked in gray. The N-terminal sequence of sStoA is as confirmed by Edman degradation. Vector pGEX4T1-encoded residues in sStoA are indicated in italics. The predicted transmembrane segments of StoA and ResA are in bold letters. Stars indicate the active site residues Cys-65 and Cys-68 and conserved residue Glu-71 of StoA. Secondary structural elements are indicated above the sequence. The alignment was obtained using AlignX, Vector NTI Suite 6.0.
StoA is similar in primary sequence to ResA (see Fig. 1), which is a ditopic membrane-bound TDOR that functions specifically as a reductase in cytochrome c maturation in B. subtilis and which has been well characterized (11). The soluble, membrane-extruded part of ResA has a typical thioredoxin fold augmented by an additional β-hairpin at the N-terminal end and a σ/β insertion between strandβ2 and helixα2 of the classic thioredoxin fold (12). The cysteine residues of the ResA active site exhibit unusually high pKa values (both above 8) (13), yielding very low reactivity of the cysteine residues at neutral pH. In contrast to most other TDORs that have been characterized, both thiols of the ResA active site are reactive to thiol-modifying reagents. In addition, a glutamate (Glu-80) in the vicinity of the active site has been shown to play a key role in controlling the reactivity of the enzyme (13, 14). StoA is not involved in cytochrome c synthesis and cannot functionally replace ResA. Likewise ResA cannot replace StoA in sporulation (2). Thus, StoA and ResA have distinctly different substrate specificities. Given their primary sequence similarity, it is of key importance to understand the basis of their specificity differences.
Here we report the isolation of the soluble domain of B. subtilis StoA (sStoA) and subsequent three-dimensional structure determination together with the biophysical characterization of the protein, including reduction potential and pKa values of the active site cysteines. Furthermore mutant variants of StoA with amino acid substitutions in the active site region have been studied in vitro and in vivo in B. subtilis. Common and discriminating features of StoA and ResA are discussed in the context of the distinctly different substrate specificities exhibited by these similar proteins.
EXPERIMENTAL PROCEDURES
Bacterial Strains and Growth of Bacteria—Strains used in this work are presented in Table 1. E. coli strains were grown in lysogeny broth or on lysogeny broth plates, and B. subtilis strains were grown in nutrient sporulation medium with phosphate (15) with appropriate antibiotics added as follows: ampicillin, 100 μg/ml; kanamycin, 10 μg/ml (for B. subtilis) or 50 μg/ml (for E. coli); and chloramphenicol, 3 or 4 μg/ml (for B. subtilis) and 15 μg/ml (for E. coli). Liquid cultures were grown in baffled E-flasks on a rotary shaker (200 rpm) at 37 °C.
TABLE 1.
Strains and plasmids used in this work
| Strain or plasmid | Genotype and/or relevant propertiesa | Origin or Ref. |
|---|---|---|
| E. coli | ||
| BL21 | F-ompT hsdSB(rB- mB-) gal dcm | Novagen |
| B834(DE3) | F-ompT hsdSB(rB- mB-) gal dcm met (DE3) | Novagen |
| MM294 | thi, pro, hsdR supE4 | 45 |
| TOP10 | F-mcrA Δ[mrr-hsdRMS-mcrBC] F80lacZΔM15 ΔlacX74 recA1 araD139 Δ[ara-leu]7697 galU galK rpsL endA1 nupG | Invitrogen |
| B. subtilis | ||
| 1A1 | trpC2 | Bacillus Genetic Stock Center, Columbus, OH |
| LUL20 | trpC2 stoAΩpLLE39; CmR | 2 |
| LUL30 | trpC2 Δ(ykvU-stoA)::tet; TcR | 2 |
| Plasmids | ||
| pBluescript SK(+) | Cloning vector; ApR | Stratagene |
| pCR-Blunt-II-TOPO | Cloning vector; KmR | Invitrogen |
| pDG148 | Expression vector; EmR KmR | 46 |
| pGEX4T1 | GST fusion expression vector; ApR | GE Healthcare |
| pLLE83 | pDG148 derivative containing the stoA gene; KmR | 2 |
| pLMC19 | pBADmyc-HisC derivative encoding sStoA; ApR | This work |
| pLYM001 | pBluescript SK(+) derivative containing stoA on a 2-kb fragment; ApR | This work |
| pLYM004 | pLYM001 derivative encoding C68A StoA; ApR | This work |
| pLYM006 | pLYM001 derivative encoding E71Q StoA; ApR | This work |
| pLYM009 | pLYM001 derivative encoding C65A StoA; ApR | This work |
| pLYM012 | pLLE83 variant encoding E71Q StoA; KmR | This work |
| pLYM013 | pLLE83 variant encoding C68A StoA; KmR | This work |
| pLYM015 | pLLE83 variant encoding C65A StoA; KmR | This work |
| pLYM025 | pCR-Blunt-II-TOPO containing stoA; KmR | This work |
| pLYM028 | pGEX4T1 derivative encoding GST-sStoA fusion protein; ApR | This work |
| pLYM031 | pLYM028 variant encoding GST-sStoA with C65A mutation; ApR | This work |
| pLYM032 | pLYM028 variant encoding GST-sStoA with C68A mutation; ApR | This work |
| pLYM033 | pLYM028 variant encoding GST-sStoA with E71Q mutation; ApR | This work |
ApR, CmR, EmR, KmR, and TcR indicate resistance to ampicillin, chloramphenicol, erythromycin, kanamycin, and tetracycline, respectively.
Construction of Plasmids Encoding sStoA—For production of sStoA plasmid pLMC19 was constructed by amplifying part of the stoA gene using oligonucleotides LE051 and LE052 (supplemental Table S1), Phusion polymerase (Finnzymes), and B. subtilis 1A1 chromosomal DNA as template. The PCR product was cloned into pCR®-Blunt-II-TOPO® (Invitrogen). The insert was cut out from the plasmid using PstI and HindIII and ligated into pBADmyc-HisC cut with the same enzymes resulting in plasmid pLMC19. The cloned stoA fragment was verified by DNA sequence analysis.
For production of a thrombin-cleavable GST-sStoA fusion protein, a fragment of the stoA gene encoding residues 21-165 of StoA was first amplified by PCR as above using oligonucleotides LY001 and LY002 as primers and subsequently cloned into pCR-Blunt-II-TOPO generating pLYM025, which was verified by sequencing. pLYM025, propagated in E. coli strain MM294, was digested by BamHI and SalI, and the stoA fragment was cloned into pGEX4T1, resulting in pLYM028.
For the construction of plasmids encoding mutant StoA variants, pLLE83 was digested by HindIII and BamHI, and the 2-kb fragment containing stoA was cloned in pBluescript SK(+), resulting in pLYM001. Site-directed mutagenesis was carried out with the QuikChange II kit and protocol (Stratagene) using pLYM001 and primers LY003-LY008 to generate plasmids pLYM009 (C65A), pLYM004 (C68A), and pLYM006 (E71Q), respectively, which were verified by sequencing. The HindIII/BamHI fragment of each of these three plasmids was subsequently cloned into pDG148, generating, respectively, pLYM015, pLYM013, and pLYM012, which were used for expression of full-length mutant stoA genes in B. subtilis. Plasmids encoding GST-sStoA fusion protein with C65A or C68A in sStoA were obtained by first amplifying stoA as above using primers LY001 and LY002 and pLYM009 or pLYM004 plasmid DNA, respectively, as template. PCR products were then cloned into pGEX4T1 as described above for the wild-type variant, generating plasmids pLYM031 and pLYM032, which were verified by sequencing.
Purification of sStoA—Non-tagged sStoA, which was utilized in initial crystallization trials and to generate a StoA antiserum, was purified from E. coli TOP10/pLMC19 as described in the supplemental data. For the production of GST-sStoA fusion protein, E. coli BL21/pLYM028 was grown in 1-liter portions in 5-liter E-flasks. At A600 = 0.6-0.8 expression was induced by addition of 1 mm isopropyl β-d-thiogalactoside (final concentration). After incubation for 5 h, cells were collected by centrifugation, washed in PBS (140 mm NaCl, 2.7 mm KCl, 10 mm Na2HPO4, 1.8 mm KH2PO4, pH 7.3), and stored as pellets at -20 °C until required. The cell pellet from 1 liter of culture was suspended in 20 ml of ice-cold PBS containing 1 mm DTT and lysed by passage (three times) through a French pressure cell at 18,000 p.s.i. The lysate was centrifuged at 48,000 × g for 40 min at 4 °C, and the supernatant was centrifuged at 100,000 × g for 1 h at 4 °C. The final supernatant was mixed with 2 ml of 50% slurry of glutathione-Sepharose 4B (GE Healthcare), and the GST-sStoA fusion protein was purified according to the resin manufacturer's instructions. Affinity-purified GST-sStoA fusion protein was cleaved by 50 units of thrombin (GE Healthcare) at room temperature for 5 h and then loaded onto a Sephacryl S-100 HR gel filtration column. Protein was eluted using 20 mm Tris-HCl, pH 8.0, containing 100 mm NaCl and 1 mm DTT. Fractions containing sStoA were identified using SDS-PAGE and Western blot with StoA antiserum, pooled, and concentrated. The N-terminal amino acid residue sequence of the purified protein was verified by Edman degradation (see Fig. 1). Cysteine variants of sStoA were produced in E. coli BL21 containing pLYM031 or pLYM032 and purified as described for wild-type sStoA.
For the production of selenomethionine-labeled sStoA, E. coli B834(DE3)/pLYM028 was grown overnight in 10 ml of SelenoMet™ medium (AthenaES) supplemented with 50 μg/ml methionine. The overnight culture was used to inoculate 1 liter of SelenoMet medium containing 50 μg/μl methionine to an A600 of 0.1, and the culture was grown until A600 was ∼0.8. Cells were harvested by centrifugation for 10 min at 10,000 × g at 4 °C, and the pellet was resuspended in 1 liter of non-supplemented SelenoMet medium and incubated for 2 h. Selenomethionine was then added to a final concentration of 50 μg/ml, and the culture was incubated for a further 30 min when production of GST-sStoA was induced by the addition of 1 mm isopropyl β-d-thiogalactoside (final concentration). Four hours after induction the culture was harvested by centrifugation for 10 min at 10,000 × g at 4 °C. The cells were washed in cold PBS and stored as a pellet at -20 °C. Selenomethionine-labeled sStoA was purified as described above. MALDI mass spectrometry confirmed that selenomethionine incorporation was close to 100%.
Crystallization and Structure Determination of sStoA—sStoA was crystallized using the sitting drop vapor diffusion method. A 2-μl sitting drop was formed by mixing equal volumes of protein solution (12 mg/ml sStoA in 25 mm Mops, pH 7.0) and crystallization reagent (27% (w/v) PEG 2000, 0.2 m ammonium acetate, 100 mm sodium acetate, pH 4.8) over an 800-μl reservoir of the reagent alone. Crystals grew over a period of 1-2 days and were cryoprotected in a solution of 30% (w/v) PEG 2000, 100 mm sodium acetate, pH 4.8, 20% (v/v) ethylene glycol before flash freezing in liquid nitrogen. X-ray data sets for native and selenomethionine-labeled sStoA were collected on beam line ID23-1 of the European Synchrotron Radiation Facility (Grenoble, France). Structure determination utilized programs of the CCP4 (16) and PHENIX (17) software suites. Diffraction patterns were indexed and integrated with MOSFLM (18) and scaled with SCALA (19). Selenium sites were identified using a combination of automated methods implemented in PHENIX.HYSS and manual inspection of anomalous difference maps produced with FFT. An initial electron density map was obtained using SAD phases calculated with MLPHARE and subsequent density modification with DM. A key factor in producing an interpretable electron density map was the identification of the correct non-crystallographic symmetry relating each sStoA molecule in the asymmetric unit. Non-crystallographic symmetry averaging in the phase improvement procedure benefited from the use of a predefined protein mask derived from a monomer of ResA (12). Further phase improvement was obtained by cross-crystal averaging with a second SAD-phased selenomethionine data set composed of merged data from two individual sStoA crystals. Manual model building was conducted in COOT, and initial phased refinement of the model was conducted with REFMAC (20). Further refinement of the model (against a single selenomethionine data set with a “low” twin fraction (α = 0.36)) utilized PHENIX.REFINE, which was essential for proper refinement of the twinned data. The final model of sStoA is composed of seven ordered protein chains and 99 water molecules. A small amount of residual density located at a coordinate of (42.34, 64.52, 19.53) may indicate the presence of an additional StoA monomer of low occupancy and high mobility that is insufficiently well resolved to enable further model building. The coordinates and structure factors have been deposited at the Protein Data Bank with accession code 3ERW.
Reduction Potential Determination—sStoA (0.2 μm) in 50 mm potassium phosphate, pH 7, was added to 5 mm oxidized DTT in the same buffer to obtain the fully oxidized protein. The protein was subsequently titrated with reduced DTT in the same buffer, allowing 10 min for the protein to equilibrate to each new potential. The transition from oxidized to reduced protein was monitored by the increase in tryptophan fluorescence emission at 344 nm (excitation at 280 nm) measured at 25 °C using a PerkinElmer Life Sciences LS-55 fluorescence spectrometer with 10-nm excitation and emission slits. Intensity was corrected for dilution effects. From the data at 344 nm, midpoint reduction potentials were determined as described previously (11, 21); further details are given in the supplemental data.
pH Stability and Cysteine pKa Measurements—Reduced wild-type and variant sStoA protein stocks were prepared in 10 mm Mops, pH 7, 2 mm TCEP (Pierce) and subsequently diluted (30-fold to a final protein concentration of 0.15 μm) with PCTC (potassium phosphate, sodium citrate, Tris, and Ches, all at 50 mm) buffer (pre-prepared at the appropriate pH) and equilibrated in a sealed cuvette for 1 h before measurement of tryptophan fluorescence spectra as above. For pKa measurements, reduced protein solutions (wild-type sStoA and single cysteine variants) were prepared in 10 mm Mops, pH 7, with 2 mm TCEP as reductant. Reaction with 6-bromoacetyl-2-dimethylaminonaphthalene was carried out under pseudo-first order conditions, and pKa values were determined as described previously (13). Further details are also given in the supplemental data.
Modification of sStoA with MAL-PEG—Wild-type sStoA and C65A and C68A variant proteins in 20 mm Tris-HCl, 100 mm NaCl, pH 8.0 were treated with 1 mm TCEP at room temperature for 30 min. Excess TCEP was removed using a YM10 column (Millipore), and each reduced sStoA sample (10 μg) was incubated with 0.1 mm or 1 mm monomethyl polyethylene glycol 5000 2-maleimidoethyl ether (MAL-PEG) (≥90%; Fluka) at room temperature for 30 min. The samples were then applied directly onto an SDS-polyacrylamide gel.
Antisera and Western Blot Analysis—Non-tagged sStoA was used to immunize rabbits (custom polyclonal antibody production service; MedProbe, Oslo, Norway). For Western blot analysis, proteins were separated by SDS-PAGE using the Schägger and von Jagow (22) system and subsequently electroblotted to a polyvinylidene difluoride membrane (Millipore) by wet blot using 20 mm Tris, 150 mm glycine, 20% (v/v) methanol. Transfer conditions were 30 V, 0.1 A overnight at 4 °C. The membrane was blocked using 5% (w/v) nonfat dry milk in 0.1% (v/v) Tween 20 in PBS. StoA antiserum was used at 1500-fold dilution. Bound primary antibodies were detected using horseradish peroxidase-linked anti-rabbit antiserum from donkey (GE Healthcare) diluted 3000-fold. Immunodetection was carried out by chemiluminescence using SuperSignal West Pico substrate (Pierce) and an Eastman Kodak Co. image station.
Preparation of Cell-free Extracts from B. subtilis Strains—Samples of 200 ml were taken from a 1.5-liter culture at time points spanning from 1 h before entry into postexponential (T = -1) to 5 h into stationary growth phase (T = 5). Cells were harvested by centrifugation; immediately washed in 50 mm potassium phosphate, pH 8.0; and frozen as a pellet at -20 °C. When required, cell pellets were thawed and suspended in 0.8 ml of phosphate buffer containing 0.7 mg/ml lysozyme, 25 μg/ml DNase, 25 μg/ml RNase, 4 mm MgSO4, and Complete protease inhibitor (Roche Applied Science; one tablet/50 ml of buffer)). After incubation at 37 °C for 45 min, an aliquot was frozen for subsequent analysis of total cell lysate. The remaining main part of the lysate was centrifuged at 48,000 × g for 60 min at 4 °C: the supernatant was used for the analysis of the soluble cell fraction, whereas the pellet, after washing in phosphate buffer, was suspended in 0.3 ml of the buffer and used for the analysis of the membrane fraction.
Other Methods—Chromosomal DNA from B. subtilis was isolated according to Marmur (23). E. coli was transformed by electroporation (24). Plasmid DNA was isolated using Quantum miniprep (Bio-Rad) or by CsCl density gradient centrifugation. SDS-PAGE was carried out using the NuPAGE system (Invitrogen) or Schägger and von Jagow (22) system. Protein concentrations were determined by measuring the absorbance at 280 nm using an extinction coefficient of 15,460 ± 100 m-1 cm-1 determined as described previously (25) or using the BCA reagent (Bio-Rad) with bovine albumin as reference.
N-terminal sequencing was carried out by Edman degradation (Protein Analysis Center, Karolinska Institutet, Sweden) on proteins separated by SDS-PAGE and electroblotted onto a polyvinylidene difluoride membrane as described for Western blotting. The membrane was stained with 0.1% (w/v) Coomassie Brilliant Blue R-250 in 2% (v/v) acetic acid, 45% (v/v) methanol. Mass spectrometry analysis was performed using an UltraFlex-MALDI-TOF/TOF mass spectrometer (Bruker Daltonics, Coventry, UK) on samples prepared by mixing sStoA in a 1:1 ratio with a saturated solution of sinapinic acid matrix in 30% acetonitrile, 0.05% trifluoroacetic acid. 0.5 μl of this combined mixture was spotted onto a polished stainless steel target and allowed to crystallize prior to analysis. The spectrometer was externally calibrated using a two-point linear calibration through the singly and doubly charged ions of trypsinogen.
The molecular mass of sStoA was determined using high performance liquid chromatography with an Ultraspherogel SEC 3000 column (Beckman). 1.2 nmol of sStoA in 10 μl was applied to the column equilibrated with 20 mm Tris-HCl, pH 8.0, 0.15 m NaCl, 1 mm DTT and eluted in the same buffer at a flow rate of 1 ml min-1. Mass was calculated based on a calibration curve obtained using catalase (230 kDa), bovine serum albumin (67 kDa), ovalbumin (46 kDa), carboanhydrase (26 kDa), myoglobin (17.2 kDa), and horse heart cytochrome c (12.3 kDa).
RESULTS AND DISCUSSION
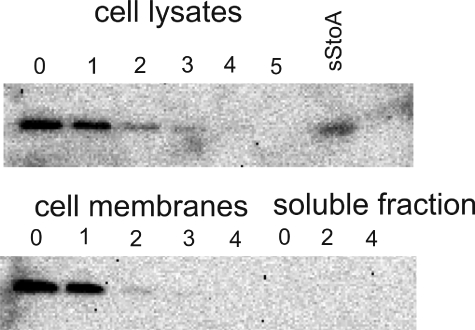
StoA Is a Membrane-bound Protein—From its amino acid sequence (Fig. 1), StoA was predicted to be a ditopic membrane-bound protein with an N-terminal ∼30-residue segment that anchors the ∼135-residue TDOR domain to the membrane. As shown previously by the use of a StoA-PhoA (alkaline phosphatase) fusion protein in E. coli cells, the transmembrane segment of StoA functions as a signal sequence to direct translocation and to membrane anchor the C-terminal TDOR domain (2). Experiments in B. subtilis indicated that the function of StoA is not dependent on the membrane anchor (3), and prediction programs suggested that the N-terminal segment might be cleaved off after the TDOR domain has been translocated across the membrane. To establish whether StoA is a membrane-bound protein, B. subtilis strain 1A1 was grown in nutrient sporulation medium with phosphate for sporulation, and samples taken at different time points during growth were analyzed for StoA by using Western blot with polyclonal antiserum directed against the TDOR domain of StoA. No StoA antigen could be detected in cell-free extracts, although BdbD, a protein very similar to StoA, was readily detected in all samples (not shown). To facilitate detection of StoA by increasing the level of the protein in cells, plasmid pLLE83 containing stoA under control of the pSpac promoter was used. StoA was found in the membrane fraction of 1A1/pLLE83 cells from early stationary phase cultures but not in late stationary phase cultures (Fig. 2). The results indicate that StoA is membrane-associated but is present in very low amounts and is degraded or trapped in maturing endospores so that it is not detectable by the Western blot procedure used.
FIGURE 2.
Subcellular localization of StoA. Western blot analysis of total cell-free lysates, membrane fractions, and soluble fractions of B. subtilis 1A1/pLLE83 for StoA antigen is shown. Cells were harvested at different time points during growth in nutrient sporulation medium with phosphate. Time point 0 is at the entry of stationary growth phase, and subsequent numbers indicate hours into stationary phase. sStoA indicates a sample of purified sStoA loaded on the gel as a reference. Approximately 20 μg of cell protein was loaded in each lane except for purified sStoA where 20 ng was loaded.
In Vivo Functional Analysis of Active Site Variants of StoA—To establish that the function of StoA in endospore biogenesis is dependent on the cysteine residues of the protein, B. subtilis strain LUL20 in which the stoA gene is inactivated and strain LUL30 in which stoA is deleted from the chromosome were used (2). These two strains containing a plasmid encoding wild-type StoA (pLLE83), C65A StoA (pLYM015), C68A StoA (pLYM013), or empty vector (pDG148) were grown for sporulation and tested for production of heat-resistant cells. Western blot analysis showed that StoA proteins were present in membranes of strains containing pLYM015 and pLYM013 (see supplemental Fig. S1). Compared with wild type, the presence of either StoA variant resulted in a ∼100-fold reduction in sporulation efficiency (Table 2). Cells completely lacking StoA, however, showed more than a 1000-fold reduction in sporulation efficiency indicating some residual activity of StoA even when one of the two cysteine residues is missing.
TABLE 2.
Efficiency of B. subtilis strains in producing heat-resistant cells after growth for 2 days at 37 °C in nutrient sporulation medium with phosphate supplemented with 1 mm isopropyl β-d-thiogalactoside
Presented are typical results obtained from at least two independent experiments with each strain, including analysis of two sister clones. B. subtilis LUL20 and LUL30 are StoA-deficient, and 1A1 is the parental strain (Table 1).
| Strain | StoA variant encoded by plasmid | Viable count before heating | Viable count after 15 min at 80 °C | Sporulation efficiencya |
|---|---|---|---|---|
| % | ||||
| 1A1 | 4.0 × 108 | 3.4 × 108 | 85 | |
| LUL20 | 5.5 × 107 | 2.0 × 104 | <0.05 | |
| LUL20/pDG148 | 4.7 × 107 | 1.2 × 104 | <0.05 | |
| LUL20/pLLE83 | Wild type | 2.6 × 108 | 1.3 × 108 | 50 |
| LUL20/pLYM015 | C65A | 4.9 × 107 | 3.7 × 105 | 0.7 |
| LUL20/pLYM013 | C68A | 7.4 × 107 | 5.0 × 105 | 0.7 |
| LUL20/pLYM012 | E71Q | 4.1 × 107 | 2.1 × 106 | 5.1 |
| LUL30 | 1.9 × 107 | 1.4 × 104 | <0.05 | |
| LUL30/pDG148 | 2.1 × 107 | 3.7 × 104 | <0.05 | |
| LUL30/pLLE83 | Wild type | 1.4 × 108 | 8.6 × 107 | 61 |
| LUL30/pLYM015 | C65A | 4.9 × 107 | 9.2 × 104 | 0.2 |
| LUL30/pLYM013 | C68A | 4.6 × 107 | 6.2 × 104 | 0.1 |
| LUL30/pLYM012 | E71Q | 3.9 × 107 | 1.6 × 105 | 0.4 |
Viable count after heat treatment divided by that before heating.
The High Resolution Structure of the Soluble Domain of StoA in a Mixture of Oxidized and Reduced States—sStoA (residues 22-165) was produced in E. coli and purified. Gel filtration analysis indicated a molecular mass of 17 ± 3 kDa (data not shown) consistent with the protein being monomeric in solution. The crystal structure of sStoA was solved using the selenomethionine SAD method of phase determination. The crystals used in structure determination belong to space group P31 and contained seven molecules of sStoA per asymmetric unit (see supplemental Fig. S2). Although structure determination was hampered by the fact that sStoA crystals were merohedrally twinned, it was nonetheless possible to refine the structure to acceptable Rwork and Rfree values using twin refinement against x-ray data obtained from a single sStoA crystal with a twin fraction (α) of 0.36. Data collection and refinement statistics for the sStoA structure are given in Table 3.
TABLE 3.
X-ray data collection and refinement statistics for sStoA
Se-Met, selenomethionine; r.m.s., root mean square. Values in parentheses represent the highest resolution shell.
| Two-crystal merged Se-Met data set | Hires Se-Met data set | |
|---|---|---|
| Space group | P31 | P31 |
| Cell parameters (Å) | a = b = 133.74, c = 64.82 | a = b = 133.72, c = 64.82 |
| Energy (eV) | 12,656.6 | 12,656.6 |
| f′ | −7.79 | −7.79 |
| f″ | 6.38 | 6.38 |
| Twinning operator | -k, -h, -l | -k, -h, -l |
| Twinning fraction | 0.38 | 0.36 |
| Resolution (Å) | 43.19-2.60 (2.74-2.60) | 36.27-2.50 (2.64-2.50) |
| Rsym | 0.154 (0.565) | 0.083 (0.293) |
| I/σ | 18.9 (4.0) | 16.1 (3.3) |
| Anomalous completeness (%) | 99.4 (96.0) | 97.2 (83.8) |
| Anomalous multiplicity | 5.0 (3.6) | 2.5 (1.7) |
| Unique observations | 44,319 (6,023) | |
| R | 0.1787 | |
| Rfree | 0.2011 | |
| r.m.s. bond (Å) | 0.040 | |
| r.m.s. angle (°) | 2.191 |
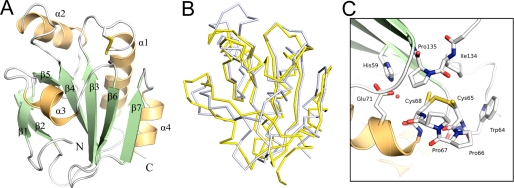
Overall the structure of each sStoA monomer (Fig. 3A) can be described as a modified thioredoxin-like fold that is highly reminiscent of B. subtilis ResA (12), CcmG (from E. coli and Bradyrhizobium japonicum) (26, 27), and Mycobacterium tuberculosis DsbE (28), which are all extracytoplasmic TDORs (see Fig. 3B). Like these proteins, the classical thioredoxin-like motif of StoA is embellished by a central αβ insertion and an N-terminal β-hairpin (in addition to the transmembrane helix predicted from primary sequence analysis). Unlike other extracytoplasmic TDORs, StoA also possesses a short insertion of residues between strand β4 and helix α2 that forms an ordered loop at the surface of the protein.
FIGURE 3.
The three-dimensional structure of the soluble domain of StoA. A, three-dimensional structure of sStoA showing that the protein exhibits a classical thioredoxin-like fold with two significant insertions: the N-terminal region contains a two-stranded, antiparallel hairpin, whereas the central insert, located after the β3-α1-β4 motif of the thioredoxin fold, comprises one helix (α2) and one strand (β5). Secondary structure elements are labeled from the N terminus (with the N-terminal transmembrane helix being 0), and the N and C termini of sStoA are indicated. B, overlay of the StoA (gray) and reduced ResA (yellow) peptide backbones (in ribbon representation). C, the active site region of StoA showing the CPPC motif, surrounding residues, and a buried water molecule (red sphere). All structural figures were prepared with PyMOL (44) and annotated with GIMP.
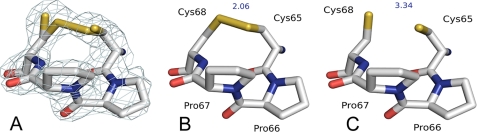
In the structure determined here, the active site cysteines of sStoA appear as a mixture of oxidized and reduced redox states (in each monomer). Crystallization of sStoA utilized solely the oxidized form of the protein, and thus it is likely that partial reduction of the disulfide bond was induced by photoreduction in the x-ray beam. The electron density associated with the partially broken disulfide is shown in Fig. 4A along with separated models of the oxidized and reduced conformations shown in Fig. 4, B and C, respectively.
FIGURE 4.
The active site of StoA in oxidized and reduced states. A, electron density (contoured at 1.2 σ) of the active site CPPC motif of StoA reveals a mixture of oxidized and reduced states. B and C, separated representations of the active site region in oxidized and reduced states, respectively. Intercysteine sulfur distances are indicated (in Å).
The oxidized (disulfide-bonded) conformation of the active site dominates the electron density and is best described as adopting a classical right-handed hook conformation with a χ3 angle of 73.5 ± 1.8° formed between the two cysteine residues. The sulfur-to-sulfur bond distance for the oxidized conformation is 2.06 Å, whereas in the reduced conformation an average sulfur-to-sulfur distance of 3.4 Å separates the cysteine residues. This distance is considerably shorter than that observed for the structure of ResA that has an exceptionally long sulfur-to-sulfur distance of 4.5 Å in the reduced state (12). Partial reduction of the disulfide does not seem to cause significant rearrangement of the local protein structure, and thus, unlike ResA, there is no evidence for any redox-linked conformational changes due to reduction of the cysteines in StoA.
In almost all known natural thioredoxin-like proteins, at least one of the two residues that intervene between the active site cysteines residues (i.e. within the CXXC motif) is a proline. In StoA, both of these intervening residues are proline. Pro-66 and Pro-67 both adopt the trans conformations and have backbone ϕ-ψ angles that are consistent with an α-helical conformation. Like all other thioredoxin-like TDORs, the CXXC motif of StoA is found at the N terminus of a reasonably long α-helix (α1 in StoA), and the macrodipole arising from this helix is often invoked as a primary cause of the lowered pKa values associated with the more N-terminal cysteine residue of the CXXC motif in most TDORs (29). The presence of proline residues at the cap of this active site helix in StoA is therefore likely to have important consequences for the distribution of the electrostatic field near the cysteines as proline does not possess a standard peptide group. Furthermore the limited conformational freedom of proline (in comparison with other residues) may be an important factor in maintaining rigidity of the CPPC motif and may be one of the reasons that the reduced form of sStoA is so similar to its oxidized form.
A further proline residue (Pro-135), which is in the cis conformation and is conserved in all thioredoxin-like proteins, is found in van der Waals contact with two buried polar residues, a histidine and a glutamate, which are located immediately behind the second cysteine of the active site motif. His-59 is located on strand β3, whereas Glu-71 arises from helix α1 directly opposite. A buried water molecule is observed in the space between these two residues within hydrogen-bonding distance of the Cys-68 sulfur (see Fig. 3C).
The arrangement of these two buried polar residues and the intervening water molecule (in StoA) is very similar to that observed in ResA where the glutamate is conserved (Glu-80) and an asparagine (Asn-68) residue takes an equivalent position to that of His-59. Substitution of Glu-80 in ResA has been shown previously to have a significant effect on the active site properties of the enzyme; for example, the pKa values of both active site cysteines were significantly lowered in an E80Q variant (13), and a B. subtilis strain containing E80Q ResA was also severely impaired in its ability to mature c-type cytochromes (14). Thus, it may be that these buried polar residues are also important in StoA function. To analyze the functional role of residue Glu-71 in StoA, the StoA-deficient B. subtilis strains LUL20 and LUL30 containing plasmid pLYM012 encoding E71Q StoA were studied. The presence of the variant StoA protein in membranes was confirmed by Western blot (supplemental Fig. S1), and the efficiency in production of heat-resistant endospores was found to be 10-150-fold (depending on strain) lower in these strains compared with wild-type controls (Table 2). This indicates that Glu-71 is functionally important in StoA.
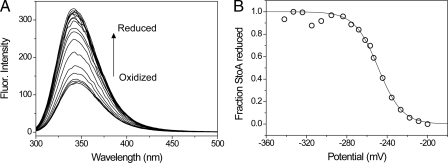
StoA Is a Low Potential TDOR—The reduction potential of the active site cysteines of sStoA was measured using the difference in tryptophan fluorescence intensity of oxidized and reduced sStoA to follow oxidation state as a function of reduction potential (see Fig. 5). The data fitted well to the Nernst equation, giving a midpoint reduction potential of -248 ± 2 mV versus normal hydrogen electrode at pH 7 with n = 2.18 ± 0.16, as expected for a two-electron reduction process. This value is similar to that measured for B. subtilis ResA (-256 mV at pH 7) (21) and E. coli thioredoxin (-270 mV at pH 7) (30, 31) and is entirely consistent with the structural similarity between these proteins and a role for StoA in the reduction of (as yet unidentified) proteins involved in endospore cortex synthesis (2, 9).
FIGURE 5.
Redox titration of sStoA. A, fluorescence spectra of sStoA in 50 mm potassium phosphate, 5 mm oxidized DTT, pH 7.0 following incubation with increasing concentrations of reduced DTT at 25 °C. B, plot of fraction of reduced sStoA (calculated from the fluorescence (Fluor.) intensity at 344 nm as described in the supplemental data) as a function of the cell potential. The solid line shows a fit to supplemental Equation S1.
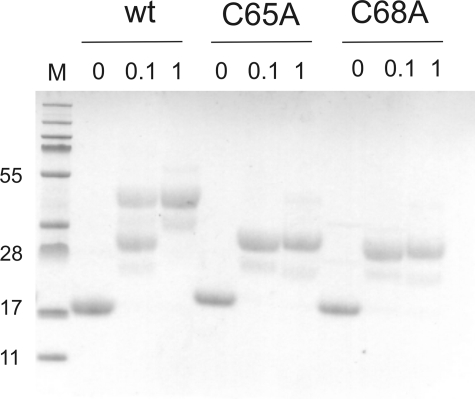
Both Active Site Cysteines Can Be Modified by Alkylating Agents—The solvent accessibility of the active site cysteines of wild-type sStoA and two variants, C65A and C68A sStoA, was investigated using MAL-PEG, a high molecular mass, cysteine-specific alkylating agent. sStoA proteins were incubated with either 0, 0.1, or 1 mm MAL-PEG as described under “Experimental Procedures” and analyzed by SDS-PAGE. Samples with exposed thiols are able to react with the MAL-PEG to form covalent complexes of significantly greater molecular mass relative to non-alkylated samples and thus are retarded during subsequent migration in the electrophoretic gel (see Fig. 6). Unmodified wild-type, C65A, and C68A sStoA variants migrated with an apparent molecular mass of ∼18 kDa, which is in reasonable agreement with the actual mass (16.4 kDa). In the presence of MAL-PEG, both single cysteine variants reacted to give a single species with a significantly lower mobility. Protein molecular mass standards cannot be used to judge the mass of MAL-PEG-modified proteins, but the significant retardation of sStoA variants is consistent with the alkylation of a single cysteine residue in each. In contrast, wild-type sStoA gave rise to two bands when incubated with 0.1 mm Mal-PEG. The lower (faster running) band corresponded to the singly modified single cysteine variants, and we conclude that under these conditions sStoA is present as a mixture of singly and doubly modified molecules. This was confirmed by incubating sStoA with a higher (1 mm) concentration of MAL-PEG that resulted in the observation of only the larger, slower running band corresponding to the doubly modified protein. In addition to these major bands, other much fainter bands were observed on the gel; these most likely arise from a small degree of heterogeneity in the size of the PEG adducts in the MAL-PEG reagent and therefore do not represent additional (non-cysteine) alkylation events or protein heterogeneity. Certainly no protein heterogeneity was observed in any of the untreated sStoA samples.
FIGURE 6.
Reactivity of the active site cysteines of sStoA. SDS-PAGE of purified reduced wild-type (wt) sStoA and C65A and C68A sStoA following reaction with MAL-PEG is shown. The protein variants were incubated with 0, 0.1, and 1 mm MAL-PEG before electrophoresis as indicated, and the gel was stained for protein. The lane indicated M contains molecular mass (kDa) markers.
From the structure it is apparent that the more N-terminal of the two active site cysteines is exposed to the solvent, whereas the other is not. Solvent-accessible surface area calculations (using a solvent probe of 1.2 Å) on the structure of reduced sStoA showed that the Cys-65 sulfur has an accessible surface area of 5.43 Å2, whereas the sulfur atom of Cys-68 is inaccessible from the bulk solvent. A similar arrangement exists in all structurally characterized thioredoxin-like proteins, and in virtually all of them, the second, buried cysteine thiol does not react with modifying reagents in solution (32, 33). This is not the case for B. subtilis ResA in which we showed previously that both cysteines are readily modified by alkylating reagents (13). To our knowledge, ResA and now StoA are the only examples where this behavior has been demonstrated. For StoA, one possibility is that alkylation of the solvent-exposed Cys-65 might cause a structural rearrangement that allows subsequent modification of Cys-68. Alternatively the reduced protein in solution may undergo dynamic motion that would allow occasional access to the sulfur of Cys-68.
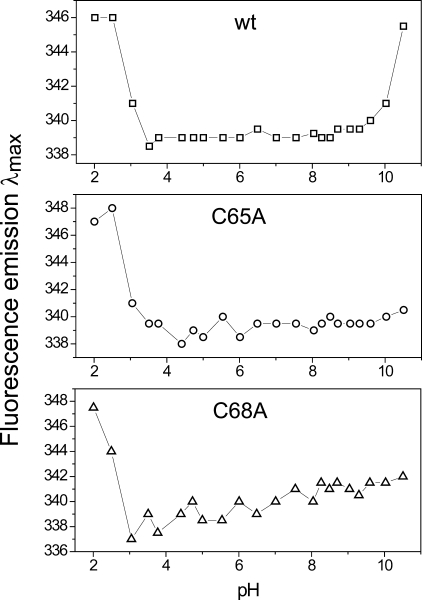
pKa Values of StoA Active Site Cysteines—The pH stability profiles of the wild-type and single cysteine variant sStoA proteins were first determined to verify the range of values over which the acid-base properties of each protein could be investigated. The intrinsic tryptophan fluorescence was measured as a function of pH for each protein under reducing conditions. Significant changes in the character of the tryptophan fluorescence emission spectrum, resulting from unfolding of the proteins, were observed at extremes of pH. Both the emission wavelength maxima and fluorescence intensity maxima were affected by pH-induced unfolding. The former has the advantage of being independent of protein concentration and was thus used preferentially in monitoring pH stability (see Fig. 7). The data showed that wild-type sStoA is stable between pH 3.5 and 9.3, whereas C65A and C68A sStoA variants are stable between 4.4 and 9.6 and between 3.7 and 9.3, respectively.
FIGURE 7.
pH stability of wild-type and variant sStoA proteins monitored by fluorescence. Plots of tryptophan fluorescence emission maxima as a function of pH for solutions of wild-type (wt) sStoA and C65A and C68A sStoA as indicated (all at 0.15 μm in PCTC buffer) as a function of pH are shown.
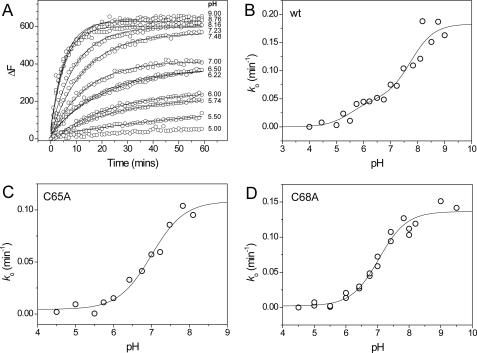
The acid-base properties of the active site cysteines of wild-type sStoA and the C65A and C68A variants were investigated by measuring rates of reaction with the fluorescent probe 6-bromoacetyl-2-dimethylaminonaphthalene as described under “Experimental Procedures” (see Fig. 8A). The fluorescence is sensitive to the environment of the modified cysteine with emission occurring in the range of ∼440-550 nm, depending on the solvent exposure of the modified cysteine (34). Here the emission maxima for Cys-65 and Cys-68 were 510 nm, indicating that the fluorescent group of both modified residues is located in a relatively solvent-exposed position. For the single cysteine variants of sStoA, data fitted well to an equation describing a single protonation/deprotonation event, giving pKa values of 7.0 ± 0.1 and 7.1 ± 0.1 for Cys-65 and Cys-68, respectively (see Fig. 8, C and D). For the wild-type protein in which both cysteine residues are intact, the data fitted well to two independent protonation/deprotonation events, giving pKa values of 5.5 ± 0.4 and 7.8 ± 0.2 (Fig. 8B). We tentatively ascribe the first transition to Cys-65 and the latter to Cys-68. Both of these values are lower than the typical value of ∼8.5-9.0 observed for cysteine, and the data are consistent with the reactivity of both residues toward alkylating reagents (which react with the deprotonated form only). A low pKa value is normally observed for the N-terminal active site cysteine of thioredoxin-like proteins, which in some cases exhibit pKa values as low as 3.5 (35). However, the pKa of the second cysteine is normally estimated to be >9 (32, 36, 37). In this respect, StoA is similar to ResA in that the C-terminal cysteinyl has a pKa value low enough to be measurable in the stable pH range of the protein.
FIGURE 8.
pKa plots for wild-type and single cysteine sStoA variants. A, time-dependent increases in fluorescence at 510 nm upon reaction of wild-type (wt) sStoA (1 μm) with 6-bromoacetyl-2-dimethylaminonaphthalene (15 μm) in PCTC buffer system at pH values from 5 to 9 as indicated at 25 °C. Plots were fitted (solid lines) to obtain an observed, pseudo-first order rate constant, ko. B, plot of ko as a function of pH for wild-type sStoA. The solid line shows a fit to supplemental Equation S4. C and D, plots of ko as a function of pH obtained from similar experiments with C65A and C68A sStoA, respectively. Solid lines represent fits of the data to supplemental Equation S3.
This large separation of pKa values is consistent with the close proximity of the two thiol groups, indicating that the ionization of one significantly influences that of the other. The wide separation of active site thiol pKa values appears to be a general feature of TDORs that act with low specificity (33, 38). For sStoA, we also observed an interdependence of the cysteine acid-base properties: the sStoA single cysteine variants have pKa values that are very similar, but in the wild-type protein they differ by more than 2 pH units. This suggests that in the wild-type protein Cys-65 and Cys-68 have a significant effect on one another such that the presence of both cysteines causes the pKa of the N-terminal cysteine to drop, whereas that of C-terminal cysteine rises (relative to the respective single cysteine variants). Such interdependence of pKa values was not observed for ResA for which respective cysteines in single cysteine variants showed acid-base properties similar to those for the wild-type protein. The stronger interdependence of the cysteine pKa values in StoA may well be linked to the significantly shorter sulfur-to-sulfur distance observed in reduced StoA (∼3.4 Å) compared with that of ResA (∼4.5 Å) (12).
Specificity Determinants of StoA and ResA—Here we have demonstrated that StoA and ResA have many features in common. The three-dimensional structure, redox properties, and acid-base properties of active site cysteine residues are similar in these proteins. Furthermore in vivo, the two proteins are believed to interact with the same integral membrane protein, CcdA, which functions to supply electrons from thioredoxin (TrxA) in the cytoplasm to the extracytoplasmic compartment (10, 39-41); thus, structural/physical features important for this interaction are expected to be shared by StoA and ResA. Despite the similarities, the two proteins do not exhibit any functional redundancy (2). So how do StoA and ResA achieve specificity for their particular substrates? To try to answer this question, it is important to identify regions of the proteins that do show differences.
First, the two proteins differ in the active site sequence motif: CPPC in StoA and CEPC in ResA. Recently we reported the effects of altering the dipeptide intervening sequence on the properties of ResA, and this included a ResA CPPC variant (21). Significant effects were observed: the redox potential increased by ∼25 mV, and the pKa values of the two cysteines decreased by 1.8 and 1.6 pH units, respectively. These findings are consistent with the midpoint reduction potential and pKa values measured here for StoA and those previously reported for ResA (13). Alteration of the dipeptide sequence was also shown to impair the in vivo activity of ResA (21). Beyond their effect on the biophysical properties of the active site cysteines (i.e. redox potential and pKa values), the intervening two residues may also be important for interaction with potential substrates. The close proximity of these residues to the active site cysteines and the fact that both are exposed on the surface of the protein make it highly likely that they contact partner proteins, at least transiently, and thus affect specificity of interaction.
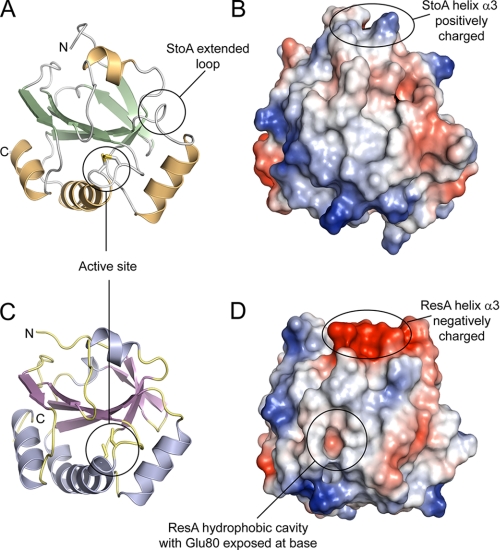
Second, the protein surfaces close to the active site are subtly different in StoA and ResA. The structures of oxidized and reduced ResA previously revealed redox-linked conformational changes, the most significant of which was the opening up of a hydrophobic cavity close to the active site upon reduction (12). Despite the conservation or conservative substitution of several of the residues that line the ResA cavity, reduction of StoA does not lead to the opening up of an equivalent cavity (see Fig. 9). The lack of a cavity in StoA may be the result of the substitution of Thr-159 (in ResA) with Pro-153 in StoA; Thr-159 undergoes one of the biggest conformational movements upon formation of the hydrophobic cavity in ResA, and thus its replacement by a proline (Pro-153) in StoA might well restrict conformational change in this region of the protein. Alternatively the lack of a cavity in StoA might be linked to the much smaller conformational change in the CXXC motif itself, which is likely to be the driving force for the larger conformational changes around the active site motif in ResA.
FIGURE 9.
A structural comparison between StoA and ResA. A and C, three-dimensional structures of sStoA and sResA, respectively, in schematic representation. B and D, surface representations of sStoA and sResA, respectively. Regions colored red indicate areas of high negative electrostatic potential, whereas blue areas indicate areas of high positive potential. Neutral regions are shown in white. Electrostatic potentials of surfaces were calculated with PyMOL. The main differences between sStoA and sResA are indicated; see the main text for details.
It has been demonstrated that Glu-80 of ResA plays a key role in vivo (14), and in vitro studies showed that it is important for the elevated pKa values of the active site cysteines and that it is capable of hydrogen bonding to amino acid side chain residues bound in the cavity (13, 21). This led us to propose that Glu-80 is important for the binding of apocytochrome substrates to ResA (12-14, 42). This residue is conserved in StoA (Glu-71), and we have shown here that it is also functionally important in StoA (Table 2). Sequence alignments showed that it is conserved in many extracytoplasmic TDORs that are proposed to have a reductive function (13). The data presented here suggest that it fulfils a similar role in ResA and StoA and also in other TDORs. This could be in controlling the acid-base properties of the active site cysteines or through direct participation in the reduction mechanism. Therefore, it is highly unlikely that this glutamate is itself an important determinant for specificity. It remains a possibility that, in ResA, it interacts directly with substrates, but this would be an additional role facilitated by the formation of the hydrophobic cavity upon reduction of the protein. Interaction of Glu-71 with substrate is not favored in StoA because the residue remains buried in both oxidation states.
Third, with the exception of the aforementioned hydrophobic cavity in (reduced) ResA, the only major difference in the electrostatic surfaces of each protein is in helix α3 (StoA numbering), which is positively charged in StoA and negatively charged in ResA (Fig. 9). However, this helix is quite distant from the active site, and it seems unlikely that this feature is responsible for differences in substrate recognition by ResA and StoA.
The final major difference is the presence in StoA of an extended loop between strand β4 and helix α2. In the primary sequence alignment (Fig. 1), this can be clearly seen as an apparent insertion/deletion of several residues that are present in the StoA but not in ResA. The sequence alignment also shows that there is little similarity between the two proteins in this region. The structure shows that the extended β4-α2 loop (composed of Ser-97, Glu-98, Gln-99, and Asn-100) lies close to the active site of StoA (Fig. 9) and represents the most significant difference in the surface shape of StoA compared with ResA and is, therefore, likely to be important for the differential substrate selectivity/specificity of these proteins. For example, this loop could be involved in specific binding interactions with StoA substrate(s) or could serve to sterically hinder interactions with non-substrate molecules.
Concluding Remarks—The structural, biochemical, and in vivo characterization of B. subtilis StoA reported here provides new knowledge about this unprecedented endospore biogenesis factor whose physiological function is not completely understood (9). Furthermore the data reveal that this low potential extracytoplasmic TDOR is remarkably similar to ResA, another well characterized extracytoplasmic TDOR from the same organism that is required for cytochrome c maturation. It is thought that both proteins interact with the same integral membrane protein, CcdA, which supplies them with electrons from the cytoplasm. The high structural similarity of ResA and StoA is no doubt connected with their shared need to interact with this protein. Despite the large extent of their similarity (in both sequence and structure), the proteins cannot functionally substitute for one another in vivo. Bacteria usually contain several thioredoxin-like proteins, soluble in the cytoplasm as well as membrane-bound. B. subtilis contains at least 10 such proteins, and none of these are essential for growth, indicating a narrow substrate specificity for each protein (43). The results of this work raise important general questions about how TDORs achieve substrate specificity: ResA can recognize at least four different apo-c-type cytochrome polypeptides as substrates, whereas StoA recognizes a different but as yet unknown substrate(s) critical for endospore cortex biosynthesis. From the structures of StoA and ResA, we have identified four principal structural differences between the two proteins that we believe provide the basis of substrate specificity/selectivity. The work demonstrates that protein-substrate specificity/selectivity can apparently be achieved through remarkably subtle variations in amino acid sequence and three-dimensional structure.
Supplementary Material
Acknowledgments
We thank Ingrid Stål for technical assistance, Dr. Allison Lewin for assistance with redox and pKa measurements, Dr. Marit Lenman for transporting a critical sample, and beam line staff at the European Synchrotron Radiation Facility for assistance in x-ray data collection.
The atomic coordinates and structure factors (code 3ERW) have been deposited in the Protein Data Bank, Research Collaboratory for Structural Bioinformatics, Rutgers University, New Brunswick, NJ (http://www.rcsb.org/).
This work was supported by a Federation of European Microbiological Societies research fellowship (to M. C. M.), by Swedish Research Council Grant 621-2007-6094, and by Wellcome Trust Grant 076017/Z/04/Z. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
The on-line version of this article (available at http://www.jbc.org) contains supplemental Equations S1-S4, Table S1, and Figs. S1 and S2.
Author's Choice—Final version full access.
Footnotes
The abbreviations used are: TDOR, thiol-disulfide oxidoreductase; Ches, N-cyclohexyl-2-aminoethanesulfonic acid; DTT, dithiothreitol; PEG, polyethylene glycol; MAL-PEG, monomethyl polyethylene glycol 5000 2-maleimidoethyl ether; Mops, 3-morpholinopropanesulfonate; TCEP, tris(2-carboxyethyl)phosphine hydrochloride; sStoA, soluble domain of B. subtilis StoA; GST, glutathione S-transferase; MALDI, matrix-assisted laser desorption ionization; TOF, time-of-flight; SAD, single wavelength anomalous dispersion.
References
- 1.Piggot, P. J., and Hilbert, D. W. (2004) Curr. Opin. Microbiol. 7 579-586 [DOI] [PubMed] [Google Scholar]
- 2.Erlendsson, L. S., Möller, M., and Hederstedt, L. (2004) J. Bacteriol. 186 6230-6238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Imamura, D., Kobayashi, K., Sekiguchi, J., Ogasawara, N., Takeuchi, M., and Sato, T. (2004) J. Bacteriol. 186 5450-5459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Raina, S., and Missiakas, D. (1997) Annu. Rev. Microbiol. 51 179-202 [DOI] [PubMed] [Google Scholar]
- 5.Dailey, F. E., and Berg, H. C. (1993) Proc. Natl. Acad. Sci. U. S. A. 90 1043-1047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meima, R., Eschevins, C., Fillinger, S., Bolhuis, A., Hamoen, L. W., Dorenbos, R., Quax, W. J., van Dijl, J. M., Provvedi, R., Chen, I., Dubnau, D., and Bron, S. (2002) J. Biol. Chem. 277 6994-7001 [DOI] [PubMed] [Google Scholar]
- 7.Yamanaka, H., Kameyama, M., Baba, T., Fujii, Y., and Okamoto, K. (1994) J. Bacteriol. 176 2906-2913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kadokura, H., Katzen, F., and Beckwith, J. (2003) Annu. Rev. Biochem. 72 111-135 [DOI] [PubMed] [Google Scholar]
- 9.Möller, M., and Hederstedt, L. (2006) Antioxid. Redox Signal. 8 823-833 [DOI] [PubMed] [Google Scholar]
- 10.Carlsson Möller, M., and Hederstedt, L. (2008) J. Bacteriol. 190 4660-4665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Erlendsson, L. S., Acheson, R. M., Hederstedt, L., and Le Brun, N. E. (2003) J. Biol. Chem. 278 17852-17858 [DOI] [PubMed] [Google Scholar]
- 12.Crow, A., Acheson, R. M., Le Brun, N. E., and Oubrie, A. (2004) J. Biol. Chem. 279 23654-23660 [DOI] [PubMed] [Google Scholar]
- 13.Lewin, A., Crow, A., Oubrie, A., and Le Brun, N. E. (2006) J. Biol. Chem. 281 35467-35477 [DOI] [PubMed] [Google Scholar]
- 14.Hodson, C. T. C., Lewin, A., Hederstedt, L., and Le Brun, N. E. (2008) J. Bacteriol. 190 4697-4705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fortnagel, P., and Freese, E. (1968) J. Bacteriol. 95 1431-1438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Potterton, L., McNicholas, S., Krissinel, E., Gruber, J., Cowtan, K., Emsley, P., Murshudov, G. N., Cohen, S., Perrakis, A., and Noble, M. (2004) Acta Crystallogr. Sect. D Biol. Crystallogr. 60 2288-2294 [DOI] [PubMed] [Google Scholar]
- 17.Adams, P. D., Grosse-Kunstleve, R. W., Hung, L. W., Ioerger, T. R., McCoy, A. J., Moriarty, N. W., Read, R. J., Sacchettini, J. C., Sauter, N. K., and Terwilliger, T. C. (2002) Acta Crystallogr. Sect. D Biol. Crystallogr. 58 1948-1954 [DOI] [PubMed] [Google Scholar]
- 18.Leslie, A. G. (2006) Acta Crystallogr. Sect. D Biol. Crystallogr. 62 48-57 [DOI] [PubMed] [Google Scholar]
- 19.Evans, P. (2006) Acta Crystallogr. Sect. D Biol. Crystallogr. 62 72-82 [DOI] [PubMed] [Google Scholar]
- 20.Murshudov, G. N., Vagin, A. A., and Dodson, E. J. (1997) Acta Crystallogr. Sect. D Biol. Crystallogr. 53 240-255 [DOI] [PubMed] [Google Scholar]
- 21.Lewin, A., Crow, A., Hodson, C. T. C., Hederstedt, L., and Le Brun, N. E. (2008) Biochem. J. 414 81-91 [DOI] [PubMed] [Google Scholar]
- 22.Schägger, H., and von Jagow, G. (1987) Anal. Biochem. 166 368-379 [DOI] [PubMed] [Google Scholar]
- 23.Marmur, J. (1961) J. Mol. Biol. 3 208-218 [Google Scholar]
- 24.Hanahan, D., Jessee, J., and Bloom, F. R. (1991) Methods Enzymol. 204 63-113 [DOI] [PubMed] [Google Scholar]
- 25.Pace, C. N., Vajdos, F., Fee, L., Grimsley, G., and Gray, T. (1995) Protein Sci. 4 2411-2423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Edeling, M. A., Guddat, L. W., Fabianek, R. A., Thony-Meyer, L., and Martin, J. L. (2002) Structure (Lond.) 10 973-979 [DOI] [PubMed] [Google Scholar]
- 27.Ouyang, N., Gao, Y. G., Hu, H. Y., and Xia, Z. X. (2006) Protein Struct. Func. Bioinformat. 65 1021-1031 [DOI] [PubMed] [Google Scholar]
- 28.Goulding, C. W., Apostol, M. I., Gleiter, S., Parseghian, A., Bardwell, J., Gennaro, M., and Eisenberg, D. (2004) J. Biol. Chem. 279 3516-3524 [DOI] [PubMed] [Google Scholar]
- 29.Kortemme, T., and Creighton, T. E. (1995) J. Mol. Biol. 253 799-812 [DOI] [PubMed] [Google Scholar]
- 30.Krause, G., Lundstrom, J., Barea, J. L., Delacuesta, C. P., and Holmgren, A. (1991) J. Biol. Chem. 266 9494-9500 [PubMed] [Google Scholar]
- 31.Lin, T. Y., and Kim, P. S. (1989) Biochemistry 28 5282-5287 [DOI] [PubMed] [Google Scholar]
- 32.Kallis, G. B., and Holmgren, A. (1980) J. Biol. Chem. 255 261-265 [PubMed] [Google Scholar]
- 33.Nelson, J. W., and Creighton, T. E. (1994) Biochemistry 33 5974-5983 [DOI] [PubMed] [Google Scholar]
- 34.Haugland, R. P., Johnson, I. D., Spence, M. T. Z., and Basey, A. (2005) Handbook: a Guide to Fluorescent Probes and Labeling Technologies, 10th Ed., Chapter 2, Invitrogen Corp., Carlsbad, CA
- 35.Huber-Wunderlich, M., and Glockshuber, R. (1998) Fold. Des. 3 161-171 [DOI] [PubMed] [Google Scholar]
- 36.Chivers, P. T., Prehoda, K. E., Volkman, B. F., Kim, B. M., Markley, J. L., and Raines, R. T. (1997) Biochemistry 36 14985-14991 [DOI] [PubMed] [Google Scholar]
- 37.Jeng, M. F., Holmgren, A., and Dyson, H. J. (1995) Biochemistry 34 10101-10105 [DOI] [PubMed] [Google Scholar]
- 38.Nordstrand, K., Aslund, F., Meunier, S., Holmgren, A., Otting, G., and Berndt, K. D. (1999) FEBS Lett. 449 196-200 [DOI] [PubMed] [Google Scholar]
- 39.Schiött, T., and Hederstedt, L. (2000) J. Bacteriol. 182 2845-2854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schiött, T., Throne-Holst, M., and Hederstedt, L. (1997) J. Bacteriol. 179 4523-4529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schiött, T., von Wachenfeldt, C., and Hederstedt, L. (1997) J. Bacteriol. 179 1962-1973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Crow, A., Le Brun, N. E., and Oubrie, A. (2005) Biochem. Soc. Trans. 33 149-151 [DOI] [PubMed] [Google Scholar]
- 43.Smits, W. K., Dubois, J. Y. F., Bron, S., van Dijl, J. M., and Kuipers, O. P. (2005) J. Bacteriol. 187 3921-3930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.DeLano, W. L. (2002) The PyMOL Molecular Graphics System, DeLano Scientific, San Carlos, CA
- 45.Amann, E., Brosius, J., and Ptashne, M. (1983) Gene (Amst.) 25 167-178 [DOI] [PubMed] [Google Scholar]
- 46.Stragier, P., Bonamy, C., and Karmazyncampelli, C. (1988) Cell 52 697-704 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.