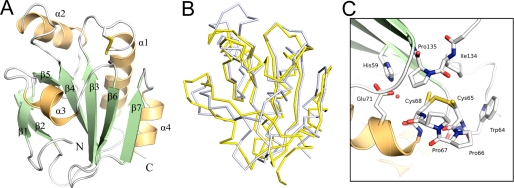
FIGURE 3.
The three-dimensional structure of the soluble domain of StoA. A, three-dimensional structure of sStoA showing that the protein exhibits a classical thioredoxin-like fold with two significant insertions: the N-terminal region contains a two-stranded, antiparallel hairpin, whereas the central insert, located after the β3-α1-β4 motif of the thioredoxin fold, comprises one helix (α2) and one strand (β5). Secondary structure elements are labeled from the N terminus (with the N-terminal transmembrane helix being 0), and the N and C termini of sStoA are indicated. B, overlay of the StoA (gray) and reduced ResA (yellow) peptide backbones (in ribbon representation). C, the active site region of StoA showing the CPPC motif, surrounding residues, and a buried water molecule (red sphere). All structural figures were prepared with PyMOL (44) and annotated with GIMP.