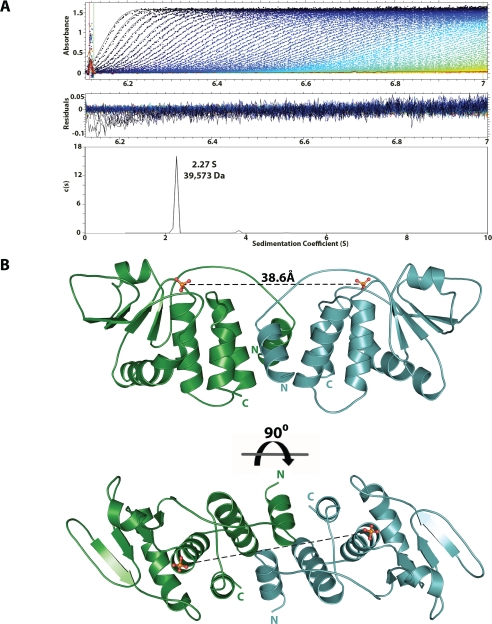
FIGURE 1.
The Vaccinia virus dual specificity phosphatase VH1 forms a dimer. A, sedimentation velocity profile of VH1 measured in 0.15 m sodium chloride at 10 °C. Top, raw absorbance at 275 nm plotted as a function of the radial position. Data at intervals of 20 min are shown as dots for sedimentation at 50,000 rpm. The monophasic sedimentation boundaries suggest that VH1 exists in a single species of homogeneous oligomeric state. Middle, the residuals between fitted curve and raw data. Bottom, the fitted distribution of the sedimentation coefficient calculated for VH1 (2.27 S and s20,w = 3.079 S) corresponds to an estimated molecular mass of ∼39,573 Da. Given the predicted size of VH1 (∼20 kDa), this indicates that in solution, VH1 exists as a dimer. B, ribbon diagram of the dimeric structure of catalytically inactive VH1 determined at 1.32 Å resolution. The distance between the two active sites in the dimer is ∼39 Å.