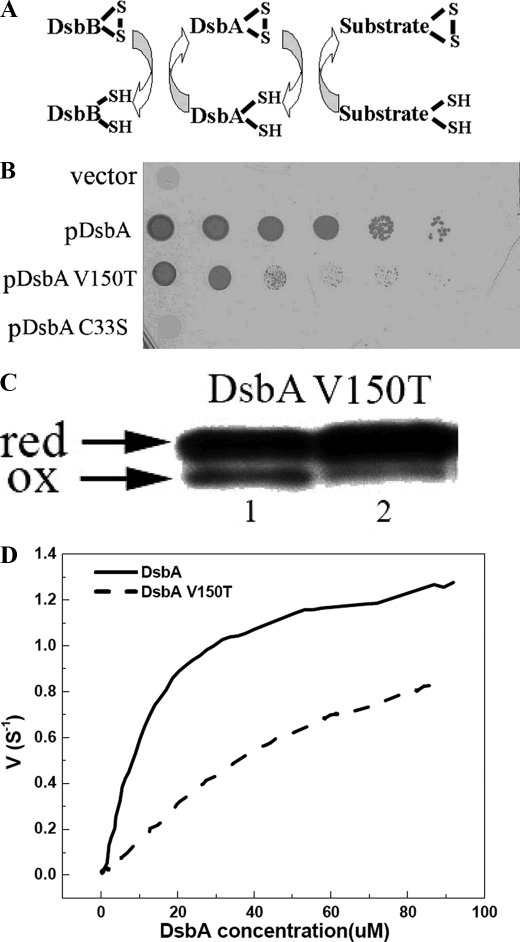
FIGURE 4.
Characterization of DsbA and mutant V150T-DsbB interaction in vivo and in vitro. A, E. coli disulfide bond formation pathway in the periplasm. B, DsbA null mutants are very sensitive to cadmium. In vivo DsbA oxidase activity was examined by measuring the cadmium resistance of DsbA null mutant strains expressing various mutants of DsbA on a plasmid. Spot titers were performed on LB plates with 40 μm cadmium. C33S, represents the DsbA active site mutant of CXXS. C, an acid trapping assay shows the slow oxidation of DsbA V150T by DsbB in vivo. Expression of DsbA and mutants was induced 10 min before AMS acid trapping. Reduced DsbA shows a 1-kDa upshift on SDS-PAGE. D, in vitro measurement of Km and kcat of DsbB catalyzing the oxidation of DsbA and variants by multi-turnover assay. The curves were fit from the average of three independent experiments. Original data are shown in supplemental Fig. S3A.