FIGURE 1.
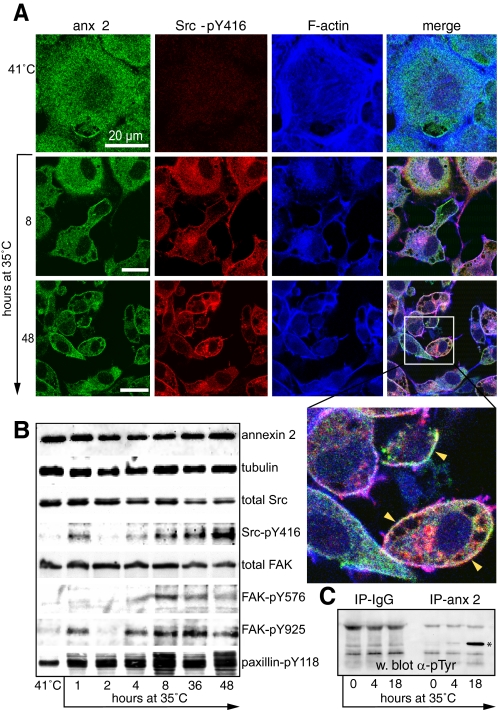
Annexin 2 and Src-pY416 relocalize during v-Src transformation of R1LA29 cells. A, R1LA29 cells were grown at the nonpermissive temperature (41 °C) and then switched to the permissive temperature (35 °C) for 8 and 48 h. Cells were stained for annexin 2 (anx 2), Src-pY416, and F-actin. The inset on the 48-h merged image shows the colocalization of annexin 2 and Src-pY416 at the plasma membrane (arrowheads). Scale bars, 20 μm. B, cell extracts were prepared from cells grown at 41 and 35 °C for the times indicated. Samples were immunoblotted for annexin 2, tubulin (as a loading control), total Src, total FAK, and also for tyrosine-phosphorylated forms of Src, FAK, and paxillin. Protein bands were visualized by enhanced chemiluminescence. C, annexin 2 is phosphorylated on tyrosine when v-Src is active. Annexin 2 was immunoprecipitated (IP-anx 2) from extracts of cells grown at 41 or 35 °C for 4 or 18 h. The samples were resolved by SDS-PAGE, Western-blotted (w. blot), and probed with antibodies to phosphotyrosine. No phosphorylated annexin 2 was immunoprecipitated by control mouse IgG (IP-IgG), and it was virtually undetectable in cells grown at 41 °C. However, a phosphoannexin 2 band was clearly visible after 4 h at 35 °C, which became prominent by 18 h (asterisk). All blots are representative of three independent experiments.