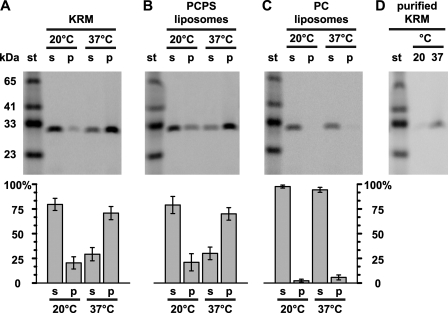
FIGURE 6.
Some membrane-bound RTA is stably embedded in the bilayer. RTA259-NBD (500 nm) was incubated with either 40 eq of KRMs (A and D), or 2.5 mm PCPS liposomes (B) in buffer H for 30 min at 20 °C or 37 °C. Membrane-bound RTA was purified by centrifugation and then extracted with alkaline sodium carbonate. The protein contents of the supernatant (s), the membrane pellet (p) fractions, and a molecular weight standard (st) were then analyzed by SDS-PAGE. C, RTA259-NBD (1 μm) was incubated with 5 mm PC liposomes in 10 mm HEPES (pH 7.5) for 30 min at 20 °C or 37 °C. The samples were then treated as in B. D, KRMs were incubated with RTA259-NBD, treated with carbonate, and then purified using a sucrose step gradient as described under “Experimental Procedures.” NBD-labeled proteins were visualized and quantified using a fluorescence imager. Representative gels from a set of at least three independent experiments are shown. Histograms show the average fraction of the total protein in the supernatant and membrane pellet fractions, respectively. The error bars indicate the S.D. of the experiments.