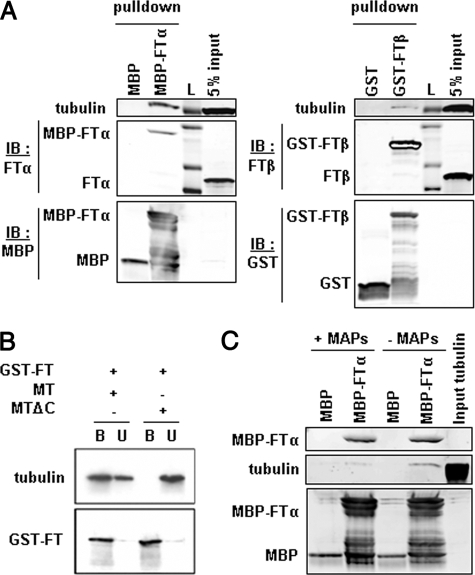
FIGURE 3.
FTase interacts directly with microtubules via its α subunit. A, FTase binding to tubulin is mediated by the FTase-α subunit. Purified MBP-tagged FTα (MBP-FTα) or MBP alone immobilized on amylase beads and GST-tagged FTase-β (GST-FTβ) or GST alone immobilized on glutathione beads were incubated with cell lysates. Bound protein fractions were resolved by SDS-PAGE and immunoblotted (IB) for the presence of FTase subunits and tubulin. No tubulin was detected in control MBP or GST beads. L, protein ladder. B, deletion of the C terminus of tubulin abolishes the interaction between FTase and microtubules. The C terminus of α- and β-tubulin in preformed microtubules was removed by proteolysis with subtilisin (MTΔC). The in vitro interaction between FTase and intact (MT) or cleaved microtubules was evaluated as in A. C, MAPs interfere with the binding of tubulin to FTase. MBP control or MBP-FTα beads were incubated with preformed MAP-containing or MAP-free microtubule protein.