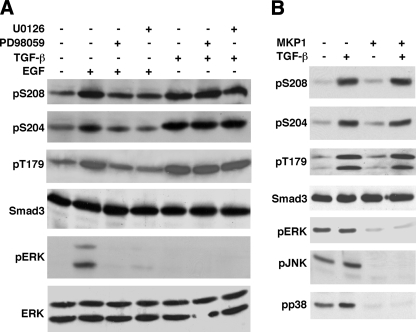
FIGURE 4.
ERK is not responsible for phosphorylation of the Smad3 linker sites in response to TGF-β. A, ERK is not the kinase that phosphorylates the Smad3 linker sites in the presence of TGF-β. Mv1Lu cells were pretreated with or without 3 μm U0126, 50 μm PD98059, or the dimethyl sulfoxide vehicle control for 1 h. Cells were then treated with 300 pm TGF-β for 1 h or 50 ng/ml EGF for 15 min. Phosphorylation of Ser208, Ser204, and Thr179 were analyzed by immunoblots with specific phosphopeptide antibodies. ERK activities were analyzed by the phospho-ERK (pERK) levels in the immunoblot. Smad3 and ERK expression levels were also analyzed by immunoblots as controls. B, MKP1 effectively inactivates ERK, JNK, and p38, but has little effect on the TGF-β-induced linker phosphorylation. L17 cells were transfected with TβRI in the absence or presence of the cotransfected MKP1, a MAP kinase phosphatase. Cells were treated with TGF-β for 1 h. Phosphorylation of Ser208, Ser204, and Thr179 were analyzed by immunoblots. ERK, JNK, and p38 activities were analyzed by pERK, pJNK, and pp38 levels, respectively. Smad3 expression levels were also examined as a control.