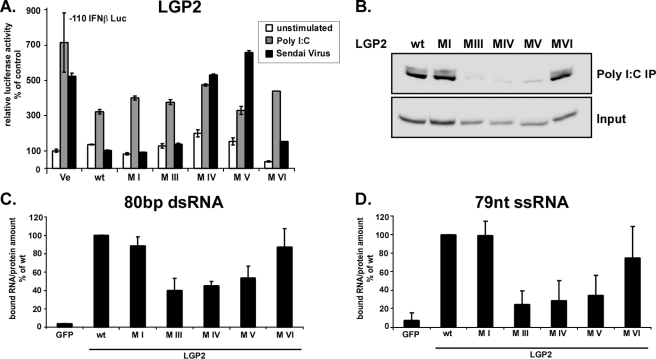
FIGURE 7.
Negative regulation by LGP2 is independent of enzymatic activity and RNA binding. A, signaling interference reporter gene assay for LGP2. Human 2fTGH cells were transfected with plasmids coding for LGP2 or mutant proteins and luciferase reporters. After 24 h, cells were left unstimulated(white) or stimulated by transfection with 5 μg of poly(I-C) (gray) or infection with 6 × 103 PFU Sendai virus (black) for 6 h and assayed for IFNβ promoter luciferase reporter gene activity. B–D, RNA binding by LGP2 and mutants. B, HEK293T cell lysate expressing LGP2 wild type or mutant protein were incubated with poly(I-C)-coated agarose beads and analyzed by immunoblot with FLAG tag-specific antiserum. C and D, LGP2 interaction with short RNA molecules. LGP2 wild type and mutant proteins were purified from HEK293T cells by immunoprecipitation with FLAG M2 affinity beads. Immobilized proteins were incubated with radioactively labeled 80-bp dsRNA (C) or 79 nt of ssRNA (D) molecules and washed extensively. Retained radioactivity was measured by scintillation counting, and counts/min are displayed as percent of wild type normalized to the total protein in each sample. Values are averaged from two independent experiments.