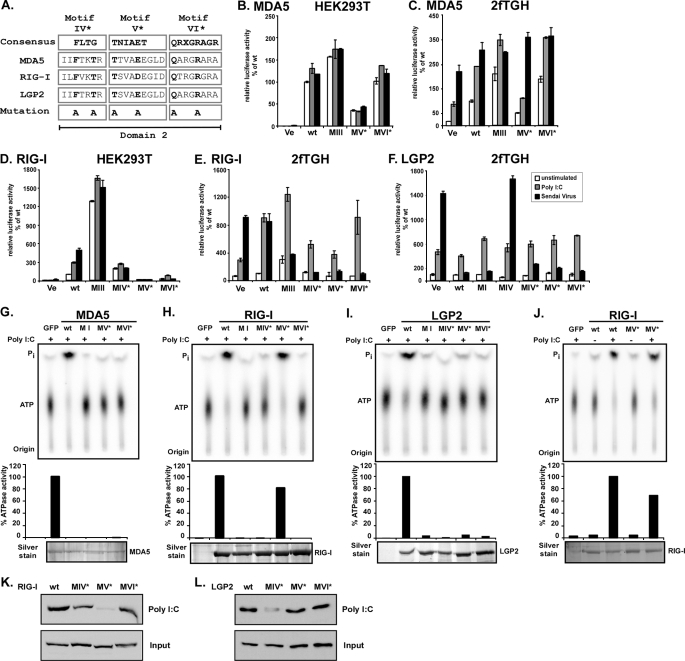
FIGURE 8.
ATP hydrolysis and antiviral signaling of point mutations in helicase domain 2. A, point mutations to helicase motifs IV–VI. The DEXH box family domain 2 consensus sequences (33) are aligned with corresponding sequences of MDA5, RIG-I, and LGP2. X indicates no amino acid preference at this position. Residues targeted for mutation are indicated in boldface type and were substituted with alanine (A). Introduced mutations are identical for all three helicases. B–F, constitutive and inducible activation of the IFNβ promoter luciferase reporter gene by expression of the wild type and mutant MDA5, RIG-I, and LGP2 proteins. HEK293T (B and D) and 2fTGH (C, E and F) cells were transfected with luciferase reporter gene plasmids and expression vectors for helicase proteins MDA5 (B and C) or RIG-I (D and E) or LGP2 (F). Parallel samples were left unstimulated (white) or were transfected with 5 μg/ml poly(I-C) (gray) or infected with 3 × 106 PFU Sendai virus (black), strain Cantell, for 6 h prior to lysis and luciferase assay. Values are normalized to the unstimulated wild type protein activity (B–E) or vector control (F) for each experiment. Representative experiments of two independent experiments are shown; error bars depict standard deviation of triplicate samples. Ve, vector control; wt, wild type. G–J, FLAG-tagged wild type and mutant MDA5 (G), RIG-I (H and J), and LGP2 (I) proteins were expressed in HEK293T cells and immunoaffinity-purified with FLAG M2 affinity gel. Eluted proteins were incubated with [γ-32P]ATP in the presence or absence (J) of poly(I-C) and separated by thin layer chromatography (top). Origin and migration of free phosphate (Pi) and ATP are indicated. ATP hydrolysis activity was quantified by phosphorimage analysis and plotted as percentages of wild type protein activity (center). Bottom panels show silver-stained SDS-PAGE demonstrating similar amounts of purified proteins in reactions. K and L, HEK293T cell lysate expressing FLAG-tagged RIG-I (K) or LGP2 (L) wild type or mutant protein were incubated with poly(I-C)-coated agarose beads and analyzed by immunoblot with FLAG tag-specific antiserum. GFP, green fluorescent protein.