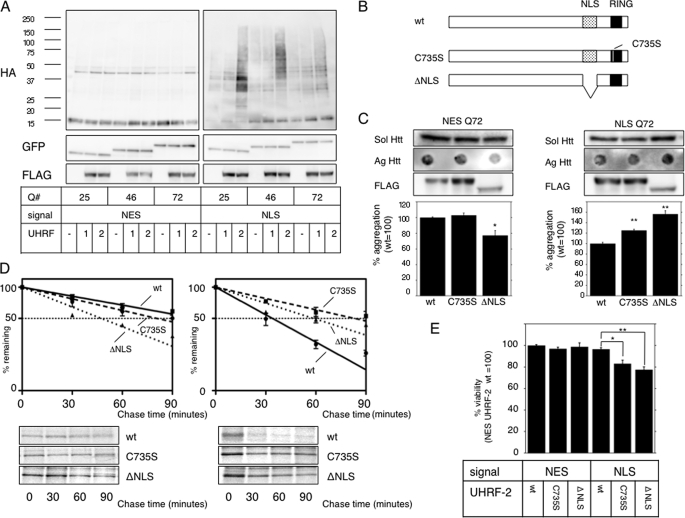
FIGURE 2.
UHRF-2 shows specific E3 activity for nuclear pQ. A, UHRF-2 ubiquitylates nuclear Htt in NES- or NLS-stable HeLa cells. FLAG-tagged UHRF-1 (1), UHRF-2 (2), or empty vector (–) was cotransfected with HA-tagged ubiquitin to NES or NLS HeLa cells for 48 h. After an additional 4 h of 5 μm MG-132 treatment, cell lysates were immunoprecipitated by anti-GFP antibody and subjected to immunoblots by anti-HA antibody (upper panels). This was reblotted by anti-GFP antibody to show immunoprecipitated monomeric Htt-GFP (middle panels). Lower panels show UHRF-1 or UHRF-2 expression in pre-immunoprecipitated lysates. B, schematic diagram showing the wt and mutants of UHRF-2. The RING domain (black box) is at its carboxyl terminus, and one of the critical cysteine residues is at amino acid 735, which was mutated to disrupt ligase activity (C735S). ΔNLS is a nuclear localization signal-defective mutant, from which amino acids 602–693 (dotted box) were deleted. C, wt UHRF-2 decreases aggregate burden. NESQ72 (left) or NLSQ72 (right) cells were transfected with wt, C735S, or ΔNLS mutants of UHRF-2 and evaluated for filter-trapped Htt-GFP. The middle panels show expression of the UHRF-2s detected by anti-FLAG antibody (FLAG). Bars indicate ±S.E. *, p = 0.0149; **, p < 0.0001 versus wt. D, UHRF-2 accelerates Htt-GFP-Q72 degradation. wt, RING domain mutant (C735S), or ΔNLS UHRF-2 was overexpressed in NESQ72 or NLSQ72 cells and a pulse-chase experiment was performed after 48 h. Relative band intensities from SDS-PAGE were normalized to 100% at time = 0 and plotted onto semi-log charts with fitted lines (wt: straight line; C735S: broken line; ΔNLS: dotted line). Bars indicate ±S.E. E, UHRF-2 rescues pQ toxicity. Htt-GFP-NES or NLS with 25Q or 97Q was transiently coexpressed with wt or C735S UHRF-2 in 293T cells. After 48 h, cell viability was measured by colorimetric assay (relative to NES plus wt UHRF-2; n = 12). Bars indicate ±S.E.; *, p = <0.01; **, p < 0.001.