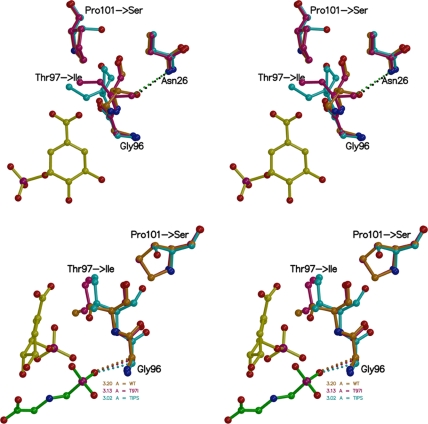
FIGURE 8.
Mutation-induced structural changes in EPSPS (stereo views). Top: EPSPS in complex with S3P. In the WT enzyme binary complex (shown in orange) Thr97 is in hydrogen bonding distance (2.8 Å) to the amide side chain of Asn96. In the T97I enzyme binary complex (maroon) the presence of the Pro101 ring holds Ile97 in place, and its side chain moves only slightly. In the TIPS enzyme binary complex (cyan) Ser101 allows larger conformational freedom for Ile97, and the isoleucine side chain swings away from Asn26. Bottom: EPSPS in complex with S3P and glyphosate bound. In the ternary complex, the mutations cause a shift of the Cα atom of Gly96 toward the phosphonate moiety of glyphosate, seen most drastically in the TIPS enzyme (cyan), thereby narrowing the inhibitor binding site. The view is ∼90° clockwise from the top.