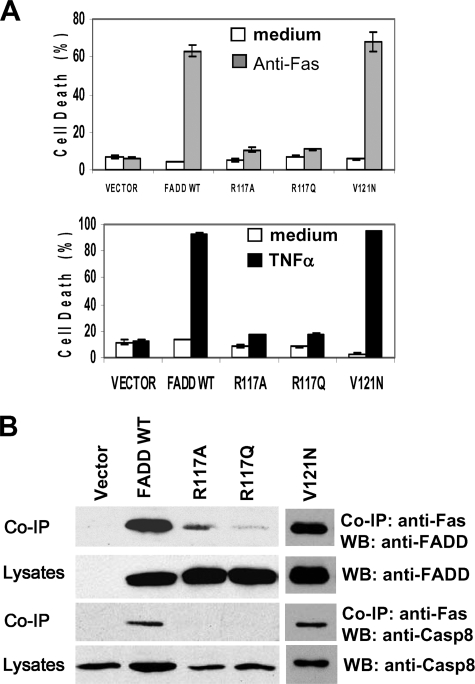
FIGURE 2.
Mutations targeting Arg-117 in the DD disrupted the apoptosis function of FADD. A, Arg-117 was replaced with glutamine, which has a side chain structure similar to that of arginine. The resulting R117Q FADD mutant was stably expressed in FADD–/– MEFs, and apoptosis was induced by anti-Fas antibodies (top) or TNFα (bottom). Similar to MEFs reconstituted with the previously described R117A mutant (21), MEFs expressing the R117Q mutant are highly resistant to apoptosis induced by Fas or TNFα. The Faslpr-like mutant of FADD, V121N, was used as a control, which is functional in apoptosis signaling. B, to detect signal-specific protein interactions, reconstituted MEFs were stimulated with anti-Fas antibodies, and Fas was immunoprecipitated (IP). After SDS/PAGE and blotting, the presence or absence of WT, R117A, R117Q, and V121N mutant FADD proteins as well as Caspase (Casp)8 in the coimmunoprecipitation complex was detected by Western blotting (WB) with anti-FADD or anti-caspase 8 antibodies.