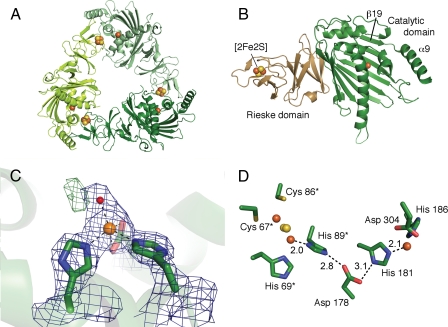
FIGURE 3.
Crystal structure of KshA from M. tuberculosis. A, ribbon representation of the KshA trimer viewed along the 3-fold symmetry axis. Monomers are colored in different shades of green. Iron ions and acid-labile sulfur atoms are shown as orange and yellow spheres, respectively. B, ribbon representation of the KshA monomer. The Rieske domain is labeled and represented in light brown, and the catalytic domain is labeled and shown in green. The location and position of the β-strand β19 and terminal helix α9 are indicated. C, mononuclear iron coordination sphere. The ribbon representation of KshA is shown as a semitransparent background. The residues ligating the iron are shown as stick representations. The mononuclear iron and solvent molecule S1 are shown as orange and red spheres, respectively. 2Fo – Fc electron density (blue mesh) is shown at 1.7 σ. Residual Fo – Fc density green mesh is shown at 3.5 σ. D, active site and adjacent Rieske center of KshA. The distance between atoms involved in proposed electron transfer is indicated. Residues from the adjacent molecule are indicated with an asterisk. Metal centers are shown as spheres, and side chains are labeled and shown as stick diagrams.